Journal of Ocular Biology
Download PDF
Research Article
* Address for Correspondence: Konstantin Galichanin, Gullstrand lab, Section of Ophthalmology, Dept. of Neuroscience, Uppsala University Hospital, SE-751 85 Uppsala, Sweden, Tel: +46 18 611 3716; Fax: +46 18 50 48 57; E-mail: konstantin.galichanin@neuro.uu.se
Citation: Galichanin K, Yu Z, Söderberg P. Upregulation of GADD45α, TP53 and CASP3 mRNA Expression in the Rat Lens after In vivo Exposure to Sub-threshold Dose of UVR B. J Ocular Biol. 2014;2(1): 5.
Copyright © 2013 Galichanin K, et al. This is an open access article distributed under the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 2, Issue: 1
Submission: 17 February 2014 | Accepted: 26 May 2014 | Published: 30 May 2014
Reviewed & Approved by: Dr. Vasantha Rao, Department of Ophthalmology and Pharmacology, Duke University School of Medicine, USA
The means, above and below the black line at 1 rel. unit, respectively, represent up- and downregulation, of the GADD45α gene.
Bartlett’s test demonstrated homoscedasticity among different post-exposure groups (test statistic = 4.57; χ 2 3;0.95 = 7.81). An analysis of variance did not elucidate any contrasts among post-exposure times (Test statistic = 2.31, F 3;32;0.95 = 2.90). A further analysis of the mean square for contrasts was analyzed with orthogonal comparisons and indicated that there is no difference in fold change in p53 mRNA expression between exposed and unexposed lenses when comparing 1 h versus 5 h (Test statistic = 2 x 10 -3 , F 1;32;0.95 = 4.15), or when comparing 24 h versus 120 h (Test statistic = 0.9, F 1;32;0.95 = 4.15). However the comparison 1 and 5 h versus 24 and 120 h, indicated a difference (Test statistic = 6.06, F 1;32;0.95 = 4.15).

Bartlett’s test demonstrated homoscedasticity among different post-exposure groups (test statistic = 4.17; χ 2 3;0.95 = 7.81). Therefore, orthogonal tests were used to analyze the contrast for fold change in CASP3 expression between exposed and unexposed lenses, among post-exposure intervals. For this, the last two values of 5 h and the last one of 24 h and 120 h post-exposure intervals, respectively, were excluded to obtain balanced sample sizes for post-exposure intervals.
The observed increase of CASP3 after 1 kJ/m 2 ( Figure 3 ) started earlier than was observed for in vivo exposure to 8 kJ/m 2 300 nm [ 20 ] ( Table 1 ) and reached approximately the same activity at 120 h with a similar increase rate. Hence, at higher dose both the TP53 response and the CASP3 response are delayed but the TP53 response precedes the CASP3 response.
Upregulation of GADD45α, TP53 and CASP3 mRNA Expression in the Rat Lens after In vivo Exposure to Sub-threshold Dose of UVR B
Konstantin Galichanin 1,2* , Zhaohua Yu 1 and Per Söderberg 1
- 1 Gullstrand lab, Section of Ophthalmology, Department of Neuroscience, Uppsala University, Uppsala, Sweden
- 2 St. Erik’s Eye Hospital, Dep. of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
* Address for Correspondence: Konstantin Galichanin, Gullstrand lab, Section of Ophthalmology, Dept. of Neuroscience, Uppsala University Hospital, SE-751 85 Uppsala, Sweden, Tel: +46 18 611 3716; Fax: +46 18 50 48 57; E-mail: konstantin.galichanin@neuro.uu.se
Citation: Galichanin K, Yu Z, Söderberg P. Upregulation of GADD45α, TP53 and CASP3 mRNA Expression in the Rat Lens after In vivo Exposure to Sub-threshold Dose of UVR B. J Ocular Biol. 2014;2(1): 5.
Copyright © 2013 Galichanin K, et al. This is an open access article distributed under the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 2, Issue: 1
Submission: 17 February 2014 | Accepted: 26 May 2014 | Published: 30 May 2014
Reviewed & Approved by: Dr. Vasantha Rao, Department of Ophthalmology and Pharmacology, Duke University School of Medicine, USA
Abstract
Purpose: The objective of the present study was to investigate the evolution of mRNA expression of the stress sensor GADD45α, the apoptosis initiator TP53 and the apoptosis executor CASP3 in the rat lens after in vivo exposure to sub-threshold dose of UVR-B. Methods: Forty six-week-old female albino Sprague-Dawley rats were unilaterally exposed to a sub-threshold dose, 1 kJ/m 2 (1.1 W/m 2 for 15 min), of UVR (λmax = 300 nm). Anesthetized and dilated eyes were exposed to UVB radiation. The animals were sacrificed at 1, 5, 24 and 120 h post-exposure. mRNA expression of the GADD45α, TP53 and CASP3 genes in the lenses was measured by quantitative RT-PCR, and fold change in mRNA expression between exposed and unexposed lenses was calculated.
Results: mRNA expression for GADD45α increased to a 1.2 fold change at 1 h after exposure and then returned to no change at 120 h. mRNA expression for TP53 increased with a regression coefficient of 0.04 h -1 to a maximum of 1.67 fold change. mRNA expression for CASP3 increased with a regression coefficient of 4.5 x 10 -3 rel. units/h to a 1.46 fold change at 120 h after exposure.
Conclusions: A sub-threshold in vivo exposure to UVR-B causes a transient upregulation of the stress sensor GADD45α at 1 h after exposure, a saturating upregulation of TP53 and a subsequent constant upregulation of CASP3 in the rat lens.
Keywords
Lens; UVR; Sub-threshold; Dose; GADD45α; TP53; CASP3; ApoptosisIntroduction
Age-related cataract is the leading cause of blindness in the world [ 1 ]. Experimental [ 2-4 ] and epidemiological [ 5-7 ] studies associate exposure to UVR-B and cortical cataract. A cross-sectional Chesapeake Bay study of 838 watermen found a link between the degree of solar UVR-B exposure and human cortical cataract [ 8 ]. Subjects with cortical cataract had a 21% higher average annual exposure to UVR-B from the age of 16, indicating that damage to the lens is cumulative. A recent experimental study in rats concluded that repeated daily in vivo sub-threshold exposures to UVR-B for 30 days partly cumulate and cause cataract in rats [ 9 ].One of the mechanisms involved in cataract formation in humans and animals is apoptosis [ 10 ]. UVR-B induces apoptosis in lens epithelial cells in vitro [ 11 ]. Transmission electron microscopy studies showed UVR-B induced apoptosis in the lens in vivo [ 12,13 ]. Proteins p53 and caspase 3 are critical in apoptosis.
p53 is a protein encoded by the TP53 gene. Caspase 3 is a protein encoded by the CASP3 gene. Both p53 and caspase 3 are involved in apoptosis as initiator and executor, respectively [ 14 ]. Active p53 binds to DNA and initiates transcription of genes involved in cell cycle arrest, DNA repair, genomic stability and apoptosis [ 15 ]. Caspase 3 is a main cysteine protease that acts as an apoptosis executor protein. Caspase 3 activates the endonuclease CAD to degrade DNA and proteases to degrade cell proteins. In molecular biology, caspase 3 is used as reliable marker of apoptosis with better sensitivity than TUNEL staining [ 16 ].
Expression of p53 and caspase 3 has been studied in the lens. Caspase 3 mRNA is expressed in untreated mouse lens fiber cells [ 17 ]. Ayala et al. showed that double threshold dose of 8 kJ/m 2 of UVR-B increases mRNA and protein expression of both p53 and caspase 3 in the rat lens epithelium 7 d after exposure [ 18 ]. Moreover, a recent study in our group concluded that expression of active caspase 3 protein increases with a peak of 16 h after exposure to sub-threshold dose of 1 kJ/m 2 of UVR-B [ 19 ]. Galichanin et al. investigated the time evolution of GADD45α, TP53 and CASP3 gene expression in the rat lens after in vivo exposure to double threshold dose of 8 kJ/m 2 of UVR-B [ 20 ]. The study concluded that UVR-B at 8 kJ/m 2 induced initial downregulation of TP53 and CASP3 mRNA messages followed by upregulation of TP53 and CASP3 mRNA messages. The authors suggested that the initial downregulation of TP53 and CASP3 reflected a depressed mRNA synthesis due to exposure to a high dose of UVR. In the current experiment, we aimed to preclude the depression of mRNA synthesis by reducing the radiant exposure dose.
GADD45α is a protein in the GADD45 gene family of genotoxic stress sensors [ 21 ]. GADD45α arrests the cell cycle at the G2/M checkpoint in order to allow repair of DNA damage. If the DNA damage cannot be repaired, cells undergo apoptosis. Thus, one expects the upregulation of GADD45α before the upregulation of TP53 and CASP3. Galichanin et al. found that GADD45α increased transiently in a time window 5 h to 24 h after in vivo exposure of the lens to UVR-B [ 20 ]. The authors suggested that UVR-B dose of 8 kJ/m 2 was high enough to cause abundant DNA damage in the rat lens, so GADD45α mRNA message increased transiently later than 5 h postexposure. In the current study of a sub-threshold dose, we hypothesize that mRNA expression of GADD45α increases earlier than 5 h postexposure.
Thus, the objective of the present study was to investigate the time course of the mRNA expression of the stress sensor GADD45α, and the apoptosis markers TP53 and CASP3 in the rat lens after in vivo exposure to a sub-threshold dose of UVR-B.
Materials and Methods
AnimalsThe six-week-old albino Sprague-Dawley (SD) female rat was the experimental animal (Taconic, Denmark). All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research. Ethical approval was obtained from the Uppsala Ethics Committee on Animal Experiments, protocol number C29/10.
Exposure to ultraviolet radiation UVR source: UVR-B at 300 nm (UVR-300 nm) was generated with a high-pressure mercury arc lamp (model 6828; Oriel, Stratford CT). The radiation was collimated, passed through a water filter and focused on the entrance slit of a double monochromator. The emerging radiation had a spectral distribution centered at 300 nm with dual peaks at 297.5 nm and 302.6 nm with 10.2 nm full width at half maximum [ 22 ].
The intensity of UVR was measured with a thermopile (model 7101; Oriel, Stratford CT) calibrated to a US National Institute of Standard traceable source.
UVR exposure: Fifteen minutes before the exposure, the animal was anesthetized with a mixture of 90 mg/kg ketamine and 10 mg/kg xylazine injected intraperitoneally and was placed in a rat restrainer [ 22 ]. Then, tropicamide, 10 mg/ml, was instilled in both eyes to induce mydriasis. Each animal was unilaterally exposed to a sub-threshold dose of 1 kJ/m 2 of UVR-300 nm during 15 minutes [ 23 ], while the contralateral eye was shielded during the exposure.
After a pre-determined latency period, the rat was sacrificed by carbon dioxide asphyxiation, followed by cervical dislocation. The eyes were enucleated and the lenses were extracted. Remnants of the ciliary body were removed from the lens equator under a microscope, keeping the lens in balanced salt solution (BSS; Alcon, USA).
RNA preparation and cDNA synthesis
From each whole lens, RNA was isolated using NucleoSpin RNA II (Macherey-Nagel GmbH & Co, Duren, Germany).
Sufficient removal of DNA from the analyte was checked with PCR using p53 DNA specific primers, forward 5′-ACCCTCTGACCTTTTTCCCA-3′ and reverse 5′-TGCTGGGATCTTAGGCACTC-3′ (biomers.net GmbH, Ulm, Germany), and Taq DNA polymerase (dNTPack). The expected PCR product was 243 base pairs. None of the analytes revealed any DNA specific PCR products on 1.5% agarose gel electrophoresis.
The RNA concentration in the analyte was measured as absorbance in a NanoDrop ND-1000 spectrophotometer (NanoDrop Products, Wilmington, DE, USA), and 1 μg of total RNA was reverse-transcribed to cDNA (1 st strand cDNA synthesis kit, Roche Diagnostics GmbH, Mannheim, Germany).
Real time PCR analysis
The cDNA made from lens RNA was analyzed in triplicates by quantitative real-time PCR on an iCycler MyiQ Single Color Real Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). The TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA, USA) was used with the TaqMan assays for gadd45a (Rn99999121_m1), p53 (Rn00755717_m1), caspase 3 (Rn00563902_m1), and 18s (Hs99999901_s1), according to the manufactures instructions. Primary fluorescence measurements were fitted with the MyiQ (Bio-Rad Laboratories, Hercules, CA, USA) algorithm and threshold fluorescence was selected standardized for each PCR plate by the algorithm. The number of cycles at threshold fluorescence was used as the measurement in MyiQ software. The PCR efficiency was estimated to 100%. The expression level of the target genes was calculated according to the Pfaffl’s mathematical model [ 24 ]. First, the difference between the threshold number of cycles of the target cDNA and the internal control 18s cDNA was calculated. Then, the difference in threshold number of cycles between exposed and non-exposed lenses was obtained. Finally, the fold change in mRNA expression of the target genes, E, was calculated by raising 2 to the power of the difference in threshold number of cycles between exposed, C exp , and non-exposed lenses, C contr (Eq. 1).
Experimental design
Altogether 40 rats were randomly divided into four post-exposure groups; 1, 5, 24 and 120 h, of ten rats. One eye in each animal was exposed in vivo to UVR-300 nm. Samples from all lenses were processed for quantitative RT-PCR of GADD45α, TP53, and CASP3 mRNAs. Additionally, the 18s rRNA was used as a reference for each lens sample. Finally, the fold change in expression of the target genes was calculated.
Statistical parameters
The significance level and the confidence coefficient were set to 0.05 and 0.95, respectively, considering sample size.
Results
mRNA expression of the GADD45α genemRNA expression of the genome stress sensor GADD45α gene is increased at 1 h after UVR exposure as evaluated from a 95% confidence interval for the mean difference, CI μ:d (0.95) = 1.20 ± 0.17 (d.f = 9), and then returns to no change at 120 h, CI μ:d (0.95) = 1.05 ± 0.18 (d.f. = 9) ( Figure 1 ).
Figure 1: Evolution of GADD45α mRNA expression in the crystalline lens after in vivo exposure to 1 kJ/m 2 UVR at 300 nm. Error bars are 95% confidence intervals for mean fold change in GADD45α mRNA expression between exposed lens and contralateral unexposed lens (d.f. = 9 for 1, 24 and 120 hrs and 8 for 5 h).
An attempt to elucidate contrast among the post-exposure intervals with orthogonal comparisons did not reveal any contrasts.
mRNA expression of the TP53 gene
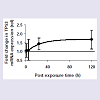
mRNA expression of the apoptosis initiator TP53 gene increased as a function of post-exposure time ( Figure 2 ).
Figure 2: Evolution of TP53 mRNA expression in the crystalline lens after in vivo exposure to 1 kJ/m 2 UVR at 300 nm. Error bars are 95% confidence intervals for mean fold change in TP53 mRNA expression between exposed lens and contralateral unexposed lens (d.f. = 9 for 1, 24 and 120 hrs and 8 for 5 h). The bold black line corresponds to the best non-linear fit.
It is plausible that there is a time delay before the onset of p53 mRNA expression followed by an increase with an increase rate that declines proportionally towards an asymptote maximum of mRNA expression. For this reason the p53 mRNA expression as a function of time, E (rel.), was fitted with non-linear regression assuming an asymptote maximum mRNA expression, E Max (rel.) and a regression coefficient, k (h -1 ).
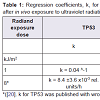
The asymptote maximum was estimated to 1.67 rel. units, the regression coefficient to 0.04 h -1 ) and the time delay for onset to 3 h.
mRNA expression of the CASP3 gene
Two of the samples from the 1 h interval and one sample from the 24 h and the 120 h interval, respectively, were lost during the sample handling and are thus excluded from the data analysis. mRNA expression of the apoptosis executor CASP3 geneincreased as a function of post-exposure time ( Figure 3 ).
Figure 3: Evolution of CASP3 mRNA expression in the crystalline lens after in vivo exposure to 1 kJ/m 2 UVR at 300 nm. Error bars are 95% confidence intervals for mean fold change in CASP3 mRNA expression between exposed lens and contralateral unexposed lens (d.f. = 7 for 1 h, 9 for 5 h and 8 for 24 h and 120 h). The bold black line corresponds to the best linear fit.
An analysis of variance did not elucidate any contrasts among post-exposure times (Test statistic = 2.44, F 3;28;0.95 = 2.95). A further analysis of the mean square for contrasts was analysed with orthogonal comparisons and indicated that there is no difference in fold change in CASP3 mRNA expression between exposed and unexposed lenses when comparing 1 h versus 5 h (Test statistic = 0.07, F 1;28;0.95 = 4.20), or when comparing 1 and 5 h versus 24 h (Test statistic = 0.01, F 1;28;0.95 = 4.15). However, when comparing 1, 5 and 24 h versus 120 h, there was a difference (Test statistic = 7.22, F 1;28;0.95 = 4.15). The experimental data were fit to a linear model for the post-exposure interval 5 to 120 h and a 95% confidence interval for the regression coefficient and the intercept, was estimated to 4.5 ± 3.0 x 10 -3 rel·h -1 and 9.3 ± 2.2 x 10 -1 rel., respectively.
Discussion
The study was designed to investigate whether exposure to a subthreshold dose of UVR-300 nm induces transcription of the stress sensor gene, GADD45α, the apoptosis initiator gene, TP53, and the apoptosis executor gene, CASP3, in the rat lens in vivo .The sub-threshold dose of 1 kJ/m 2 was chosen to be substantially below the threshold dose for UVR-B induced cataract in six-week-old SD rats, expressed as MTD 2.3:16 [ 25 ], to assure that no significant light scattering was expected after the UVR exposure.
The current finding that there is a slight increase of GADD45α 1 h post-exposure that subsequently seems to decrease asymptotically ( Figure 1 ) indicates that, at sub-threshold dose for induction of light scattering, there is a GADD45α response. When we exposed the animal to a double threshold dose, no such increase was detectable [ 20 ] probably due to too extensive toxic effects of the UVR exposure.
The presently observed increase of GADD45α mRNA message at 1 h ( Figure 1 ) that precedes the increase of TP53 and CASP3 mRNA messages is consistent with the previous investigations showing that GADD45α encodes a protein that serves as a genome stress sensor [ 21 ]. GADD45α protein activates the G2/M cell cycle arrest to allow the cell to repair the damage. If the damage cannot be repaired, the cell is eliminated through apoptosis. The current finding that the initial upregulation of stress sensor GADD45α is followed by the upregulation of apoptosis markers TP53 and CASP3 in the rat lens after in vivo exposure to UVR-B supports this scheme.
Our present observation that TP53 increased exponentially declining towards an asymptote ( Figure 2 ) is consistent with our previous finding that TP53 increases after in vivo exposure to UVR 300 nm [ 20 ]. However, the response onset is delayed at the higher dose compared to the lower dose ( Table 1 ).
Such a finding of less time to onset of mRNA expression in the current study, as compared to the previous investigation of lenses exposed to 8 kJ/m 2 [ 20 ], can be explained by the absence of initial depression of mRNA synthesis seen in the previous study.
Caspase 3 protein is used as a reliable marker of apoptosis with better sensitivity than TUNEL staining [ 16 ]. Recently, Talebizadeh et al. showed that expression of active protein caspase 3 increases in the lens after in vivo exposure to sub-threshold dose of UVR-B of 1 kJ/m 2 with the peak between 8 h and 16 h followed by the decrease towards 24 h [ 19 ]. However, present results revealed increased mRNA expression of CASP3 in exposed lenses at later time interval of 120 h after exposure to 1 kJ/m 2 of UVR-B. Such a discrepancy in kinetics between mRNA expression and protein expression of caspase 3 in the lens can be explained by the presence of cortical fiber cells expressing mRNA message of CASP3 at 120 h after UVR-B exposure and few apoptotic lens epithelial cells expressing active caspase 3 protein at 24 h. Further, mRNA synthesis of CASP3 might not be required just after UVR exposure since there is enough pool of protein caspase 3 in the cells to start apoptosis.
There is data showing that sub-threshold dose of 1 kJ/m 2 does not induce significant lens light scattering [ 26 ]. However, the present data showed that in vivo exposure to 1 kJ/m 2 UVR induces increased mRNA expression of TP53, CASP3 and GADD45α genes. Thus, the current data indicates that there are molecular biology responses after exposure to UVR at 1 kJ/m 2 even if increased light scattering does not develop.
The time gap between the onset of TP53 mRNA expression, 3 h, and the onset of CASP3 mRNA expression, 17 h, was calculated to be 14 h. This is in concordance with the common scheme of apoptosis suggesting that it takes several hours between activation of p53 protein and caspase 3 protein [ 14 ].
Apoptosis was detected in cataractous human and rat lenses [ 10 ]. Authors suggested that if a strong defence system in the lens exists, it repairs UV damage and produce few apoptotic cells. However, if the defence system is weakened, it cannot repair UV damage sufficiently and thus, produce many apoptotic cells that accumulate over time to probably initiate human cataract. So, in response to DNA damage that cannot be repaired, p53 initiates the transcription of the genes that encode the pro-apoptotic Bcl2 proteins, which trigger the mitochondrial pathway of apoptosis. Mitochondrial pathway ends at the execution pathway that is known by the cleavage of caspase 3 that activate endonuclease to degrade DNA and proteases to degrade proteins in the cell. Ayala et al. studied the expression of apoptosis markers p53 and caspase 3 on mRNA and protein levels in lenses exposed to single dose 8 kJ/m 2 of UVR-B in vivo and found that exposed lenses exhibit increased expression of both [ 18 ]. Our findings of mRNA expression of p53 and caspase 3 in the rat lens after in vivo exposure to UVR-B are almost in accordance with the previous results of Ayala et al. However, in this study we used lower subthreshold single dose of UVR-B, 1 kJ/m 2 . Further investigations are required to study apoptosis markers’ expression in the cataract model of repeated sub-threshold exposures to UVR-B, which simulates agerelated cataract in humans.
In conclusion, a sub-threshold dose of 1 kJ/m 2 UVR-B causes a transient upregulation of the stress sensor GADD45α at 1 h after exposure, a saturating upregulation of TP53 that precedes a constant upregulation of CASP3 in the rat lens in vivo . It is foreseen that the present results will be useful for future investigations of the UVR induced cataract at the mRNA and the protein levels.
Acknowledgements
The paper was presented as an abstract at the European Association for Vision and Eye Research Congress 2011. This work was supported by the Karolinska Institutet KID-funding, Karolinska Institutet Eye Research Foundation, Gun och Bertil Stohnes Stiftelse, Karin Sandqvists Stiftelse, Konung Gustav V:s och Drottning Victorias Frimurarstiftelse The Uppsala university/Uppsala Läns Landsting’s ALF Research grants.References
- Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, et al. (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82: 844-851.
- Pitts DG, Cullen AP, Hacker PD (1977) Ocular effects of ultraviolet radiation from 295 to 365 nm. Invest Ophthalmol Vis Sci 16: 932-939.
- Söderberg PG (1990) Experimental cataract induced by ultraviolet radiation. Acta Ophthalmol 1-75.
- Galichanin K (2012) Ultraviolet Radiation Cataract. Thesis: Karolinska Institutet 1-56.
- Cruickshanks KJ, Klein BE, Klein R (1992) Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health 82: 1658-1662.
- Delcourt C, Carriére I, Ponton-Sanchez A, Lacroux A, Caovacho MJ, et al. (2000) Light exposure and the risk of cortical, nuclear and posterior subcapsular cataracts: Pathologies Oculaires Liees a l’Age (POLA) study. Arch Ophthalmol 118: 385-392.
- McCarty CA, Taylor HR (2002) A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Dev Ophthalmol 35: 21-31.
- Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, et al. (1988) Effect of ultraviolet radiation on cataract formation. N Eng J Med 319: 1429-1433.
- Galichanin K, Löfgren S, Söderberg P (2014) Cataract after repeated daily in vivo exposure to ultraviolet radiation. Health Physics [Not Published]
- Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, et al. (1995) Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol 130: 169-181.
- Li WC, Spector A (1996) Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med 20: 301-311.
- Michael R, Vrensen GF, van Marle J, Gan L, Söderberg PG (1998) Apoptosis in the rat lens after in vivo threshold dose ultraviolet irradiation. Invest Ophthalmol Vis Sci 39: 2681-2687.
- Galichanin K, Löfgren S, Bergmanson J, Söderberg P (2010) Evolution of damage in the lens after in vivo close to threshold exposure to UV-B radiation: cytomorphological study of apoptosis. Exp Eye Res 91: 369-377.
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495-516.
- Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8: 275-283.
- Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, et al. (2003) Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol 199: 221-228.
- Zandy AJ, Lakhani S, Zheng T, Flavell RA, Bassnett S (2005) Role of the executioner caspases during lens development. J Biol Chem 280: 30263-30272.
- Ayala M, Strid H, Jacobsson U, Söderberg PG (2007) p53 expression and apoptosis in the lens after ultraviolet radiation exposure. Invest Ophthalmol Vis Sci 48: 4187-4191.
- Talebizadeh N, Yu Z, Kronschläger M, Söderberg P (2014) Time evolution of active caspase-3 labelling after in vivo exposure to UVR-300 nm. Acta Ophthalmol [Epub ahead of print].
- Galichanin K, Svedlund J, Söderberg P (2012) Kinetics of GADD45α, TP53 and CASP3 gene expression in the rat lens in vivo in response to exposure to double threshold dose of UV-B radiation. Exp Eye Res 97: 19-23.
- Liebermann DA, Hoffman B (2008) Gadd45 in stress signaling. J Mol Signal 3: 15.
- Galichanin K, Löfgren S, Bergmanson J, Söderberg P (2010) Evolution of damage and repair of the lens after in vivo close to threshold exposure to UV-B radiation: cytomorphological study of apoptosis. Exp Eye Res 91: 369-377.
- Ayala MN, Michael R, Söderberg PG (2000) Influence of exposure time for UV radiation-induced cataract. Invest Ophthalmol Vis Sci 41: 3539-3543.
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45.
- Söderberg PG, Löfgren S, Ayala M, Dong X, Kakar M, et al. (2002) Toxicity of ultraviolet radiation exposure to the lens expressed by maximum tolerable dose. Dev Ophthalmol 35: 70-75.
- Michael R, Söderberg PG, Chen E (1998) Dose response function for forward light scattering after in vivo exposure to ultraviolet radiation. Graefes Arch Clin Exp Ophthalmol 236: 625-629.