Journal of Ocular Biology
Download PDF
Review Article
*Address for Correspondence: Qizhou Lian, MD, PhD, Department of Medicine and Department of Ophthalmology, The University of Hong Kong, Hong Kong, China, Tel: +852-21899752; Fax: +852-28162095; E-mail: qzlian@hku.hk
Citation: Yan L, Jiang D, He J, Wong DSH, Lian Q. Limbal Stem Cells and Corneal Epithelial Regeneration: Current Status and Prospectives. J Ocular Biol. 2014;2(1): 10.
Copyright © 2013 Yan L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 2, Issue: 1
Submission: 07 March 2014 | Accepted: 26 April 2014 | Published: 02 May 2014
Reviewed & Approved by: Dr. Bjørn Nicolaissen, Department of Ophthalmology, University of Oslo, Norway
The limbal microenvironment termed “LSC niche” is first proposed by Schofield in 1983 [29]. The niche serves as a ‘reservoir’ for harboring and supporting a small population of putative LSCs, as well as providing a barrier to excessive growth of conjunctival cells and invasion of blood vessels into the cornea. The conjunctival ingrowth of the cornea occurs when the protective function fails and stable immunity of the cornea is destroyed. Environmental factors of niche include the limbal extracellular matrix, particularly the basement membrane, cell-matrix interactions, and cell-cell contacts. The underlying limbal stroma with keratocytes and blood supply network also plays a role in this environment by releasing various soluble cytokines [30-32]. In 1989, Kolega J et al. found that the basement membrane component AE-27 expressed weakly in the limbal region, and correlated with negative staining for CK3 [33]. In 1995, Ljubimov et al. gave rise to the assumption that the heterogeneity of the limbal extracellular matrix might be partially responsible for the different cell phenotypes and proliferative behavior. The limbal basement membrane is composed of collagen IV (α1- 2) chains and laminin (α1-3, β1-3, γ1-2) chains; whereas collagen type IV (α3-5) chains and laminin (α1 and 3, β1 and 3, γ1-2) chains are contained in the corneal basement membrane [34-36]. Along this line, Espana et al. found that the CK3-negative phenotype of the limbal basal cells is mediated through the limbal stroma/basement membrane complex [37]. Diverse studies collectively point out that the niche is crucial in regulating the self-renewal and fate decision of LSC, although the precise mechanism remains obscure. In 2005, compared to submerged culture, the results of limbal explants air-lifting culture suggested that epithelial-mesenchymal transition via the Wnt/β-catenin pathway influences the fate of limbal epithelial progenitor cells, between regeneration and fibrosis during wound healing when the stromal niche is activated [38].
In the first instance, conditions such as tear film deficiency, inflammation, lid abnormalities and corneal and conjunctival anesthesia should be investigated and addressed prior to surgery [66]. Use of cultured or non-cultured tissue and cell type should then be determined. Technically, the ideal method requires an easily accessible transplantation material, effective transplantation and stable clinical outcomes.
Limbal Stem Cells and Corneal Epithelial Regeneration: Current Status and Prospectives
Limeng Yan2,3, Dan Jiang2,3, Jia He2,3, David SH Wong3 and Qizhou Lian1,2,3*
- 1Department of Medicine, the University of Hong Kong, Hong Kong, China
- 2Shenzhen Institutes of Research and Innovation, the University of Hong Kong, China
- 3Department of Ophthalmology, the University of Hong Kong, Hong Kong, China
*Address for Correspondence: Qizhou Lian, MD, PhD, Department of Medicine and Department of Ophthalmology, The University of Hong Kong, Hong Kong, China, Tel: +852-21899752; Fax: +852-28162095; E-mail: qzlian@hku.hk
Citation: Yan L, Jiang D, He J, Wong DSH, Lian Q. Limbal Stem Cells and Corneal Epithelial Regeneration: Current Status and Prospectives. J Ocular Biol. 2014;2(1): 10.
Copyright © 2013 Yan L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 2, Issue: 1
Submission: 07 March 2014 | Accepted: 26 April 2014 | Published: 02 May 2014
Reviewed & Approved by: Dr. Bjørn Nicolaissen, Department of Ophthalmology, University of Oslo, Norway
Abstract
The clear cornea functions like a window that controls the entry of light for visual information and plays a protective role. The failure of appropriate repair following corneal injury results in loss of corneal function. The limbal region of the cornea is thought to serve as a unique reservoir of corneal epithelial stem cells where limbal stem cells (LSC) contributed to the regeneration of corneal epithelium. The deficiency of LSC (LSCD) results in the failed regeneration of corneal epithelium following injuries. In this review, we discuss the current knowledge of LSC and LSC-based transplantation for regeneration of corneal epithelium. We will first review the latest development of corneal structures. Next we will introduce the concept of LSC and the associated debates. Third, we will review different LSC-based transplantation methods for LSCD treatment and compare their advantages and disadvantages. Finally, we will discuss the improvements of regeneration of corneal epithelium.Keywords
Corneal structure; Limbal stem cell; Cell-based transplantation; Regeneration of corneal epitheliumAbbreviations
LSC: Limbal Stem Cell; LSCD: Limbal Stem Cell Deficiency; TAC: Transit Amplifying Cell; TDC: Terminally Differentiated Cell; CK: Cytokeratin; ABCG2: ATP-Binding Cassette Transporter G2; LEC: Limbal Epithelial Crypt; LC: Limbal Crypt; CX: Connexins; LEC: Limbal Epithelial Cell; ESC: Embryonic Stem Cell; HCjE: Conjunctival Epithelial Cell; BM-MSC: Bone Marrow-Derived Stem Cell; EpiASC: Epidermal Adult Stem Cell; IDPSC: Immature Dental Pulp Stem Cell; HFSC: Hair Follicle-derived Stem Cell; MSC: Mesenchymal Stem Cells; AM: Amniotic Membrane; CLAU: Conjunctival Limbal Autograft; KLAL: Keratolimbal Allograft; HPCLK: Homologous Penetrating Central Limbo-Keratoplasty; SLET: Simple Limbal Epithelial Transplantation; lr-CLAL: Living-related Conjunctival Limbal AllograftsIntroduction
The cornea is a transparent and avascular tissue, located at the front part of the eye. Its main function is to transmit and focus light to the correct position at the back of the eye for visual perception. Corneal tissue comprises three major layers: epithelium on the superficial surface, a stroma on the middle layer, and endothelium on the inner surface. The corneal epithelium sheds with stratified epithelial cells, and is thought to be replaced by stem cells located at the narrow edge of the cornea, in a region known as the limbus. The limbal region of the cornea provides a unique reservoir of corneal epithelial stem cells: limbal stem cells (LSC) are thought to be the main source for the regeneration of corneal epithelium following injury. A deficiency of limbal stem cells (LSCD) results in failure of corneal regeneration following injury, with consequent poor repair and ultimately loss of corneal transparency and subsequent blindness. In the past few decades, intensive research has focused on corneal stem cells as a source of regenerative cell-based therapy. This review summarizes the current knowledge of corneal epithelial stem cells and LSC-based transplantation in the regeneration of corneal epithelium. We will first review old and new information about the corneal structure. Second, we will discuss the current concept of LSCs; third, we will review studies of LSC-based transplantation and their advantages and disadvantages. Finally, we will discuss the future applications of LSCs.Corneal Structure: Old and New Discoveries
As the window of the eye, the healthy cornea remains completely transparent and plays a major role in visual information. The clear cornea covers the front of the eye and is surrounded by the outer conjunctiva and inner sclera. These enclosing structures maintain the eyeball’s globe shape and protect the internal functional content.The crucial function of vision is achieved by the highly specialized corneal structure, previously described as comprising five layers: epithelium, Bowman’s layer, stroma, Descemet’s membrane and endothelium. Transparency of the cornea is essential for clear vision and is achieved by a smooth epithelium with no encroachment of conjunctival cells. The transparency of the cornea also is associated with the absence of vasculature, uniformly spaced collagen fibers, a functional endothelium that regulates corneal hydration and the production of crystalline proteins by keratocytes in the stroma [1,2].
The smooth epithelium is the uppermost part of the whole tissue, providing a non-keratinized, stratified squamous layer. Differentiated squamous cells present microvilli (finger-like projections) and microplicae, with a glycocalyx scaffold coating the surface. These components provide the structural framework to support and bind a complex of related factors, including tears, mucus, immunoglobulins and compact junctions between the epithelial cells, and constitute a protective barrier against invasion by infection [3]. During normal blinking, dead squamous cells are sloughed from the corneal epithelium. The corneal epithelium is subject to a constant cycle of cell renewal approximately every 9-12 months [4]. Epithelial cell loss from the corneal surface was defined in the “X, Y, Z hypothesis” by Thoft and Friend in 1983 [5]. The naturally desquamated epithelial cells (Z) are constantly replaced by proliferating and dividing cells at the basal epithelium (X), and cells which can migrate to the centre from the periphery (Y).
X +Y = Z
Thus, migration occurs centripetally and circumferentially from the limbus and vertically from the basal layer forwards. Although the hypothesis is still questionable, there is a number of evidence to support this original observation. Remarkably, the animal study data supported the clinical results in LSCD patients [6-10].
The Bowman’s layer is acellular and composed of collagen fibrils, lying between the epithelium and stroma as a separate entity from the subepithelial basement membrane. This layer may be a visible indicator of ongoing stromal-epithelial interactions in the human. When stromal-epithelial interactions are disturbed in diseases, the Browman layer is commonly broken up [11]. The stroma, which constitutes up to 90% of the thickness of the cornea, is made up of bundles of regular spatial, near-uniformly thick connective collagen type I and IV fibers. The fibers are produced by keratocytes and embedded by an extracellular matrix. Stromal-epithelial interactions are bi-directional communications mediated by soluble cytokines during development, homeostasis, and wound healing in organs. The stromal-epithelial interactions in the cornea are regulated by hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF). HGF and KGF are released by the keratocytes to modulate physiologic functions of epithelial cells, like proliferation, motility and differentiation. The epithelial to stromal interactions are also modulated by interleukin-1 (IL-1) and soluble Fas ligand, which are produced by corneal epithelial cells following injury. IL-1 dominantly regulates corneal wound healing through functions such as matrix metalloproteinase, HGF and KGF production, and apoptosis of keratocytes. The Fas/Fas ligand system primarily contributes to the immune privileged status of the cornea. Other cytokines, including epithelium growth factor from keratocytes and tumor necrosis factor α from epithelial cells, also play the important roles in the interactions [12]. Besides the corneal epithelial cells [13], and keratocytes in the supporting stroma [14], there are another two sources supplying multiple cytokines in the maintenance and healing of the cornea: the adjacent tear film [15], the aqueous humor [16].
The Descemet’s membrane, which can regenerate following injury, serves as the modified basement membrane between the stroma and the endothelium. The endothelium is the innermost, single hexagon-shape cell layer of the cornea. With a different origin, function and appearance to vascular endothelium, it governs the transport of nutrients and retains the slightly dehydrated state of the cornea [17]. Compared to some mammals such as rabbits, guinea pigs and cats, the human endothelium lacks a complete regenerative capacity through cellular division and subsequent migration, which is similar to the monkey’s endothelium [18].
Concept of Limbal Stem Cells and the Associated Debates
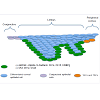
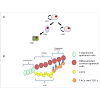
Limbus microenvironmentThe limbus is the border zone between the transparent cornea and the opaque ‘whites of the eye’. Various studies related to the limbal microenvironment suggest that this special region participates in maintaining “stemness”. Histologically, limbal epithelium is unique; it consists of more than 10 cell layers and is the thickest among the three as compared to 1-2 cell layers for the conjunctival epithelium and 4-6 cell layers for the corneal epithelium [19] (Figure 1). In humans, a regional specialization of the epithelial structure in the limbus was first speculated by Davanger and Evensen, and called “palisades of Vogt” [20]. This structure is rich in melanin content, which can protect LSC from UV damage. The basal cells of this structure, some of which are the presumed LSC, are tightly attached to the underlying basement membrane and have a rich network of blood supply through the characterized radially oriented fibrovascular ridges. The ridges are more common in the superior and inferior quadrants around the eye. The palisades of Vogt are composed of stromal invaginations, allowing access to chemical signals that diffuse from the underlying vascular network [21]. In 1986, Schermer A et al. presented a 64K keratin, cytokeratin 3 (CK3), was positively expressed in the limbal basal layer, and led strong support of LSC location in the limbus [22]. In 1989, George Cotsarelis et al. identified a subpopulation of slowcycling limbal basal cells using 3H-thymidine labeling, which shared a set of common features with various epithelial stem cells. In common with other adult somatic cells, LSCs are small in size, have a high nuclear to cytoplasmic ratio, and lack expression of differentiation markers. In the event of injury, the slow cycling LSCs become highly proliferative [23]. These putative LSCs simultaneously retain their capacity for self-renewal and maintain a constant cell number by giving rise to fast-dividing progenitor cells, termed transit amplifying cells (TAC) [24]. These TAC compose the majority of the proliferative cell population in the corneal/limbal epithelium, and can experience a limited number of divisions before turning into terminally (postmitotic) differentiated cells (TDC) [22]. The process of TDC shedding from the ocular surface during normal wear and tear stimulates epithelial cell division, migration, and differentiation [1,25] (Figure 2A and 2B). The differences between the two progenitor cells, LSC and TAC, are various, such as the expression of some markers (see 2.2), pigment protection, cell cycle length, response to tumor promoter [23,26,27] cell size, and ex vivo expansion supported material [28].
Figure 1: The illustration of limbus and surrounding epithelium region. Limbal epithelium consists of more than 10 cell layers and is the thickest among the three as compared to 1-2 cell layers for the conjunctival epithelium and 4-6 cell layers for the corneal epithelium. The upper few epithelial layers are flat-shaped. Limbus basal cells express a combination of putative ‘LSC markers’.
Figure 2: (A) A diagram denoting the self-renew and proliferative capability of limbal stem cells (LSCs). LSCs give rise to transit amplifying cells (TACs) as well as LSCs. TACs become mature and divide into terminally (post-mitotic) differentiated cells (TDCs). (B) A demonstration showing the relationship between LSC and other neighboring cells. LSCs locate in the basal layer of the limbal epithelium. LSC-derived TACs locate from the peripheral cornea to the central cornea, and gradually fail to proliferation. TDCs progressively replace the desquamated corneal differentiated epithelial cells.
Evidence of LSC
No single, definitive marker has yet been found to identify LSC despite numerous attempts. Nonetheless, the expression of a combination of ‘markers’ is used for identification and isolation of putative LSC, either positive (present) or negative (absent). Limbal basal cells lack differentiation markers such as CK3 that is present in all other layers of the corneal epithelium and the suprabasal layers of the limbal epithelium [22]. CK12 is also expressed in a similar pattern [39]. Involucrin is expressed in the corneal stratified squamous epithelium as a marker of differentiation [40]. Connexin 43 (CX43) is present in the corneal basal cells except that of the limbus for cellcell communication by gap junctions. The lack of CX43 expression in stem cells helps protect them against damage affecting adjacent neighbors [41].
Although the limbus is acknowledged as the site of corneal epithelial stem cells, the well-defined anatomical niche for LSCs was not described until 2005. Dua’s group re-evaluated the systematic serial 5-7 mm sections of human corneoscleral segments obtained from cadaver donors, with the corneal epithelial marker CK14 and the “stem cell” marker ATP-binding cassette transporter G2 (ABCG2) [42]. These distinct anatomical extensions from the limbal palisades, which consist of a solid cord of CK14/ABCG2-positive cells extending along the basal cells of the limbus more than along the basal cells of the adjacent conjunctiva, revealed features of being a LSC niche. This identified structure was termed the limbal epithelial crypt or limbal crypt [43]. Thus far, besides humans and pigs, a limbal epithelial crypt has not been discovered in other species [44].
Yang et al. first demonstrated that transcription factor p63 was critical during epithelial development [45]. Initially, p63 was considered to be a specific LSC marker in human cornea [46]. However, later studies found that p63 was involved in normal development and carcinomas [47]. The preferential expression of ΔNp63α (the α isoform of N-terminal transactivation domainnegative p63) in activated human limbal basal layer suggested that ΔNp63α was the most specific marker for LSC [48,49]. In 2007, using confocal imaging, Shortt et al. first provided the evidence in vivo that the structures of the palisades of Vogt are only one facet of the limbal niche architecture, forming the lateral walls of the stromal structures that encircle limbal crypts. Meanwhile, consistent with the previous studies [40,48], the putative LESC markers ABCG2 and ΔNp63α were expressed only by limbal basal cells and not by the corneal epithelium [50].
N-Cadherin and CK15 are two other promising candidate LSC antigens that co-localize to clusters of progenitor-like cells in the limbus [51]. The cell cycle arrest transcription factor C/EBPδ has also been implicated in the regulation of LSC self-renewal [52] (Figure 1).
The dogma that limbal stem cells are the exclusive source of corneal epithelial cells has been recently challenged. In 2008, Majo et al. found that in both mice and young children, healthy central corneal epithelium could generate holoclones with characteristics of stem cells that can maintain the corneal epithelium, probably without acute participation from the limbal region [53]. Furthermore, Dua et al. discovered normal central islands of corneal epithelium in adult eyes with clinically total limbal stem cell deficiency (LSCD) [54]. These data demonstrate that some LSCs remain and contribute to maintenance of the central epithelium in a clinically invisible way, or that basal cells of the central surviving epithelium can independently maintain the central epithelium. Nonetheless, the location of stem cells in the ocular surface may be species- or age-specific [54]. Majo et al. proposed the hypothesis that LSC functions response to injury and are not concerned with normal wear and tear of the corneal epithelium [53]. This remains to be determined by advanced studies related to the long-accepted TAC hypothesis. In addition, Dua et al. questioned whether LSC would regenerate the corneal epithelium in acute participation when the healthy central corneal islands were destroyed [54]. The first indication of the presence of LSCs was the observation in a rabbit that melanin moved centripetally from the limbus towards a corneal epithelial defect [55]. Limbal LSC transplantation following the corneal epithelium injuries in clinical practices indicated a high success rate of corneal wound healing, further supporting the hypothesis of the location of LSCs [20,56-59].
Limbal Stem Cell Deficiency and Treatment
Limbal stem cell deficiencyLSCD is characterized principally by damage or dysfunction of limbal stem cells with consequent invasion of conjunctival epithelium into the cornea [2]. The X or Y component of the X, Y, Z hypothesis is changed under such a pathologic condition, and induces failure of corneal homeostasis.
The etiology of LSCD is varied but primarily includes congenital disease, ocular trauma or disease (e.g. trachoma, pterygium), autoimmune disease (e.g. Stevens Johnson syndrome and ocular cicatricial pemphygoid), systemic disease (e.g. diabetes), chemical burn, ultraviolet or ionising radiation, iatrogenic injury and contact lens-related pathology.
The process of conjunctivalization is considered the hallmark of LSCD [60]. The other combined clinical signs are also involved, such as goblet cell infiltration, corneal neovascularization, and persistent epithelial defects and scarring. As a consequence, LSCD culminates in visual impairment and persistent pain in patients [61]. LSCD is classified as partial or total, and unilateral or bilateral [62]. If the pupillary area is covered by encroaching conjunctival epithelium, intervening action should be undertaken [63], as well as the indication from significant ocular pain.
Treatment of LSCD
A plentiful range of strategies to treat LSCD has been developed since 1940, when amniotic membrane (AM) was used in the first tissue-based procedure. Penetrating keratoplasty as the standard therapy of central corneal replacement is not available for LSCD, since restoration of the stem cell population is not involved [64], with the donor source and graft rejection as important limitations [19,65].
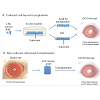
Current therapy of LSCD can be categorized using either cultured or non-cultured tissue or cell type for transplantation or alternative approaches without transplantation. In present review, we focus on the development of both cultured and non-cultured cell-based transplantation (Figure 3A and 3B).
Figure 3: (A) The experimental process of cultured cell-based transplantation in LSCD treatment. LSCs in different cell types are expanded in various ex vivo system with substrate, and harvested cells with or without substrate are transplanted onto the ocular surface of hosts. The mouse model represents ocular appearance with LSCD caused by alkali burn. Some characteristic features of LSCD, e.g. vision reduction, neovascularization, and persistent corneal epithelial defect can be observed. (B) The experimental process of non-cultured cell-based transplantation in LSCD treatment. LSC-tissue grafts from different sites of donor ocular surface are directly transplanted onto the ocular surface of hosts. The mouse model represents both normal cornea and ocular appearance with LSCD caused by alkali burn.
Cultured cell-based transplantation: In cultured LEC transplantation, LECs are expanded on substrate ex vivo from living or cadaver human cornea [67], and transplanted with or without the substrate onto the bare cornea that has been exposed by surgically removing the fibrovascular pannus (Table 1). Repeating this transplantation in the same eye can lead to higher final clinical success rate [68] and improvement in visual acuity [69]. The overall significant improvement rate of cultured LSC transplantation (according to the differently considerable criteria in different studies) was higher for autografts than for allografts [60,70,71]. The most common complications following transplantation included bleeding, inflammation, blepharitis and epitheliopathy. Less common was keratitis, residual fibrin, rejection of cultured LECs, corneal perforation, glaucoma, and infection [60,68,71-74]. Follow-up should continue for at least two years, especially since many complications are observed during the first year [68,69]. A benefit of this transplantation is that the risk of stem cell failure in the donor eye can be minimized via a small biopsy [75], with further opportunities to harvest LSCs [69,72]. Another advantage is the reduced risk of rejection of allografts compared with direct limbal tissue transplantation [76]. The major limitation is the high cost of stem cell procedures and high requirement of expertise. It remains unknown how cultured LSCs reconstruct the ocular surface. It is widely held that LECs may replace progenitor/stem cells, and/or revitalize dormant stem cells of the recipient by providing growth factors/chemotactic stimuli.
Much attention has recently focused on oral mucosal epithelial cells [72,77,78] in contrast to other cultured non-LSC cell sources. The neovascularization that occurs following oral cell transplantation may be diminished by anti-angiogenic therapy [79]. As another feasible cell source, conjunctival epithelial cells, have been applied in animal and human LSCD models in the same way as oral cells [80,81]. Other documented cultured cell types include embryonic stem cells, bone marrow-derived mesenchymal stem cells (MSCs), epidermal epithelial cells, human immature dental pulp stem cells, hair folliclederived stem cells, umbilical cord stem cells.
Non-cultured cell-based transplantation: A direct transplantation without requirements of cell culture procedures has been employed in various tissues (Table 2). Amniotic membrane (AM), the innermost layer of the placenta, serves broadly as an extracellular matrix for cell cultivation and a carrier for cultured cell transplantation. AM comprises a single layer of epithelium, a thick basement membrane and an avascular stroma and has been shown to support adhesion, migration, differentiation and proliferation of epithelial cells [97]. The dominant properties of AM substrate are low or no immunogenicity with anti-inflammatory, anti-angiogenic, and anti-scarring properties [98,99]. AM transplantation alone is not appropriate for total LSCD, but is feasible in partial LSCD therapy [100]. Recent studies have nonetheless indicated far broader applications of AM transplantation. For example, AM extract and non-cultured tissue combined with AM transplantation therapy [101,102].
Conjunctival limbal autograft (CLAU) [103] has limitations to be used for unilateral LSCD because limbal tissue attached to a conjunctival carrier from the healthy eye of the patient is required, and the transplantation process may increase the risk of LSCD in the healthy donor eye. In living-related conjunctival limbal allografts (lr-CLAL), the grafts from a living relative of the patient show a poorer long-term outcome than CLAU due to the unavoidable need for systemic immunosuppression [103-105]. Living donor allografts are nonetheless superior to cadaver allografts.Based on the different choices of allografts, homologous penetrating central limbo-keratoplasty (HPCLK) is associated with a greater risk of immune rejection than conventional penetrating keratoplasty [106], while keratolimbal allograft (KLAL) involves the transplantation of cadaveric limbal tissue with its abundance of stem cells, together with a small rim of corneal and scleral tissue [103,104]. A combination of AM or lr-CLAL and KLAL is performed in some cases [107].
Simple limbal epithelial transplantation (SLET) has been shown to be valuable in the management of human LSCD. Compared with cultured LEC transplantation, this process can notably reduce the cost and period of cell culture, using very small amounts of biopsy tissue. Further clinical data are essential for the improvement of SLET, and a novel biodegradable alternative to AM is required.
Challenges and Future Perspectives
Before corneal stem cell transplantation can be widely applied in clinical practice, a number of challenges need to be addressed. At present, most investigational protocols in corneal bioengineering and corneal stem cell therapy rely on the use of animal products and/or allogeneic human cells and tissue. Such products raise potential risks, such as graft-versus-host disease, cataract, dry eye, glaucoma as well as animal transmitted diseases. More suitable material is urgently needed. A novel method of culturing expanded human limbal epithelial cells on human AM ex vivo has been developed using a cultured medium with autologous human serum as single growth supplement. Compared to the commonly used complex medium including FBS and other non-human derived products, omission of xenogenic ingredients may reduce the host immunogenicity of the transplanted tissue and also safeguard against the inter-species transmission [108]. Sequentially, above protocol was applied in clinical trial, and five in nine transplanted patients had obviously achieved clinical improvement in 11 to 28 months follow-up [109]. From another point of view, the enzyme-related (e.g., trypsin-EDTA and dispase) dissociation or sequential incubation steps in cultured cell-based transplantation would induce DNA damage in the cell population destined for graft production. It is essential to maintain integrity of cellular and DNA repair mechanisms for proper cellular functioning including for long-term viability and proliferative potential. Therefore, it requires further optimization to improve ex vivo manipulating procedures and protocols to guarantee the quality of cell transplantation [110].It is also important to systematically assess the advantages and disadvantages of cultured versus non-cultured limbal stem cellbased transplantation. It requires more understanding of molecular signals that constitute the limbal stem cell niche. Simultaneously, considering that the reported success rate differs widely between studies and depends on various parameters including the sources of tissue and the indications for surgery/concomitant ocular pathology, it is warranted for well-defined and stringent criteria to further illuminate and evaluate the transplantation methods and therapeutic outcomes among the prospective studies. Preferably, a greater number of patients with long enough follow-up data are necessary to assess the efficiency and superiority of the cell types and technique in LSCD therapy [109]. In addition, there is no consensus on the specific markers to identify limbal stem cells making between-study comparisons difficult.
In summary, although it is urgently needed to acquire more knowledge on appropriate potential cell sources, scaffold material, and growth elements, the progress in both basic research and clinical treatments indicate that there is great hope for stem cell-based therapies for regeneration of corneal epithelium in the near future.
Acknowledgements
This research is in part supported by HKU Seed Funding Programme for Basic Research Small Project Funding (201311159115to Q Lian); National Natural Science Grant of China (31270967 to Q Lian), and Innovation and Technology Fund, Hong Kong (ITS/150/12 to Q Lian).References
- Estey T, Piatigorsky J, Lassen N, Vasiliou V (2007) Aldh3a1: a corneal crystalline with diverse functions. Exp Eye Res 84: 3-12.
- Osei-Bempong C, Figueiredo FC, Lako M (2013) The limbal epithelium of the eye--a review of limbal stem cell biology, disease and treatment. Bioessays 35: 211-219.
- Nichols B, Dawson CR, Togni B (1983) Surface features of the conjunctiva and cornea. Invest Ophthalmol Vis Sci 24: 570-576.
- Wagoner MD (1997) Chemical injuries of the eye: Current concepts in pathophysiology and therapy. Surv Ophthalmol 41: 275-313.
- Thoft RA, Friend J (1983) The x, y, z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 24: 1442-1443.
- Xu X, Mannik J, Kudryavtseva E, Lin KK, Flanagan LA, et al. (2007) Co-factors of LIM domains (Clims/Ldb/Nli) regulate corneal homeostasis and maintenance of hair follicle stem cells. Dev Biol 312: 484-500.
- Koizumi N, Rigby H, Fullwood NJ, Kawasaki S, Tanioka H, et al. (2007) Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol 245: 123-134.
- Pajooresh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA (2006) Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells 24: 1075-1086.
- Gurdal C, Takmaz T, Sargon MF, Anayol A, Ylmaz E, et al. (2006) Electron microscopic evaluation of the effect of therapeutic silicone hydrogel lenses on the limbal area. Eye Contact Lens 32: 133-137.
- Ouyang J, Shen YC, Yeh LK, Li W, Coyle BM, et al. (2006) Pax6 overexpression suppresses cell proliferation and retards the cell cycle in corneal epithelial cells. Invest Ophthalmol Vis Sci 47: 2397-2407.
- Wilson SE, Hong JW (2000) Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea 19: 417-420.
- Wilson SE, Liu JJ, Mohan RR (1999) Stromal-epithelial interactions in the cornea. Prog Retin Eye Res 18: 293-309.
- Rolando M, Zierhut M (2001) The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 45: S203-S210.
- West-Mays JA, Dwivedi DJ (2006) The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell B 38: 1625-1631.
- Watanabe K, Nakagawa S, Nishida T (1987) Stimulatory effects of fibronectin and EGF on migration of corneal epithelial cells. Invest Ophthalmol Vis Sci 28: 205-211.
- Welge-Lussen U, May CA, Neubauer AS, Priglinger S (2001) Role of tissue growth factors in aqueous humor homeostasis. Curr Opin Ophthalmol 12: 94-99.
- Waring GO 3rd, Bourne WM, Edelhauser HF, Kenyon KR (1982) The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 89: 531-590.
- Van Horn DL, Hyndiuk RA (1975) Endothelial wound repair in primate cornea. Exp Eye Res 21: 113-124.
- Tseng SC (1989) Concept and application of limbal stem cells. Eye (Lond) 3: 141-157.
- Davanger M, Evensen A (1971) Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229: 560-561.
- Goldberg MF, Bron AJ (1982) Limbal palisades of Vogt. Trans Am Ophthalmol Soc 80: 155-171.
- Schermer A, Galvin S, Sun TT (1986) Differentiation-related expression of a major 64k corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103: 49-62.
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM (1989) Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57: 201-209.
- Zieske JD (1994) Perpetuation of stem cells in the eye. Eye (Lond) 8: 163-169.
- Beebe DC, Masters BR (1996) Cell lineage and the differentiation of corneal epithelial cells. Invest Ophthalmol Vis Sci 37: 1815-1825.
- Kruse FE, Tseng SC (1993) A tumor promoter-resistant subpopulation of progenitor cells is larger in limbal epithelium than in corneal epithelium. Invest Ophthalmol Vis Sci 34: 2501-2511.
- Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SC (2003) Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci 44: 5125-5129.
- Meller D, Pires RT, Tseng SC (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol 86: 463-471.
- Schofield R (1983) The stem-cell system. Biomed Pharmacother 37: 375-380.
- Kruse FE, Tseng SC (1993) Differing regulation of proliferation of limbus and corneal epithelium caused by serum factors. Ophthalmologe 90: 669-678.
- Kruse FE, Tseng SC (1993) Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Invest Ophthalmol Vis Sci 34: 1963-1976.
- Espana EM, Kawakita T, Romano A, Di Pascuale M, Smiddy R, et al. (2003) Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci 44: 5130-5135.
- Kolega J, Manabe M, Sun TT (1989) Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation 42: 54-63.
- Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT et al. (1995) Human corneal basement membrane heterogeneity: Topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest 72: 461-473.
- Tuori A, Uusitalo H, Burgeson RE, Terttunen J, Virtanen I (1996) The immunohistochemical composition of the human corneal basement membrane. Cornea 15: 286-294.
- Fukuda K, Chikama T, Nakamura M, Nishida T (1999) Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 18: 73-79.
- Espana EM, Kawakita T, Romano A, Di Pascuale M, Smiddy R, et al. (2003) Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci 44: 5130-5135.
- Kawakita T, Espana EM, He H, Li W, Liu CY, et al. (2005) Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol 167: 381-393.
- Chaloin-Dufau C, Sun TT, Dhouailly D (1990) Appearance of the keratin pair K3/K12 during embryonic and adult corneal epithelial differentiation in the chick and in the rabbit. Cell differ Dev 32: 97-108.
- Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, et al. (2004) Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 22: 355-366.
- Matic M, Petrov IN, Chen S, Wang C, Dimitrijevich SD, et al. (1997) Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation 61: 251-260.
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7: 1028-1034.
- Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A (2005) Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Brit J Ophthalmol 89: 529-532.
- Mort RL, Douvaras P, Morley SD, Dora N, Hill RE, et al. (2012) Stem cells and corneal epithelial maintenance: insights from the mouse and other animal models. Results Probl Cell Differ 55: 357-394.
- Yang A, Schweitzer R, Sun DQ, Kaghad M, Walker N, et al. (1999) P63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398: 714-718.
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, et al. (2001) P63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 98: 3156-3161.
- Rocco JW, Ellisen LW (2006) P63 and p73: life and death in squamous cell carcinoma. Cell Cycle 5: 936-940.
- Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, et al. (2005) Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A 102: 9523-9528.
- Kawasaki S, Tanioka H, Yamasaki K, Connon CJ, Kinoshita S (2006) Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Exp Eye Res 82: 293-299.
- Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, et al. (2007) Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells 25: 1402-1409.
- Ordonez P, Di Girolamo N (2012) Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells 30: 100-107.
- Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, et al. (2007) C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol 177: 1037-1049.
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y (2008) Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 456: 250-254.
- Dua HS, Miri A, Alomar T, Yeung AM, Said DG (2009) The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology 116: 856-863.
- Mann I (1944) A study of epithelial regeneration in the living eye. Br J Ophthalmol 28: 26-40.
- Srinivasan BD, Eakins KE (1979) The reepithelialization of rabbit cornea following single and multiple denudation. Exp Eye Res 29: 595-600.
- Hanna C (1966) Proliferation and migration of epithelial cells during corneal wound repair in the rabbit and the rat. Am J Ophthalmol 61: 55-63.
- Buck RC (1979) Cell migration in repair of mouse corneal epithelium. Invest Ophthalmol Vis Sci 18: 767-784.
- Kinoshita S, Friend J, Thoft RA (1981) Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci 21: 434-441.
- Nakamura T, Inatomi T, Sotozono C, Ang LP, Koizumi N, et al. (2006) Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology 113: 1765-1772.
- Dua HS, Forrester JV (1990) The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol 110: 646-656.
- Dua HS, Saini JS, Azuara-Blanco A, Gupta P (2000) Limbal stem cell deficiency: Concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol 48: 83-92.
- Dua HS, Joseph A, Shanmuganathan VA, Jones RE (2003) Stem cell differentiation and the effects of deficiency. Eye (Lond) 17: 877-885.
- Thoft RA (1979) Conjunctival transplantation as an alternative to keratoplasty. Ophthalmology 86: 1084-1091.
- Pleyer U, Schlickeiser S (2009) The taming of the shrew? The immunology of corneal transplantation. Acta Ophthalmol 87: 488-497.
- Pellegrini G, Rama P, De Luca M (2011) Vision from the right stem. Trends Mol Med 17: 1-7.
- Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S (2001) Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 108: 1569-1574.
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, et al. (2010) Limbal stem-cell therapy and long-term corneal regeneration. N Eng J Med 363: 147-155.
- Basu S, Ali H, Sangwan VS (2012) Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol 153: 643-650.
- Nakamura T, Sotozono C, Bentley AJ, Mano S, Inatomi T, et al. (2010) Long-term phenotypic study after allogeneic cultivated corneal limbal epithelial transplantation for severe ocular surface diseases. Ophthalmology 117: 2247-2254.
- Daya SM, Watson A, Sharpe JR, Giledi O, Rowe A, et al. (2005) Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology 112: 470-477.
- Shortt AJ, Secker GA, Rajan MS, Meligonis G, Dart JK, et al. (2008) Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology 115: 1989-1997.
- Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S (2001) Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 108: 1569-1574.
- Shimazaki J, Higa K, Morito F, Dogru M, Kawakita T, et al. (2007) Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol 143: 945-953.
- Jenkins C, Tuft S, Liu C, Buckley R (1993) Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond) 7: 629-633.
- Espana EM, Di Pascuale M, Grueterich M, Solomon A, Tseng SC (2004) Keratolimbal allograft in corneal reconstruction. Eye (Lond) 18: 406-417.
- Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, et al. (2004) Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Eng J Med 351: 1187-1196.
- Shimazaki J, Higa K, Kato N, Satake Y (2009) Barrier function of cultivated limbal and oral mucosal epithelial cell sheets. Invest Ophthalmol Vis Sci 50: 5672-5680.
- Lim P, Fuchsluger TA, Jurkunas UV (2009) Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol 24: 139-148.
- Di Girolamo N, Bosch M, Zamora K, Coroneo MT, Wakefield D, et al. (2009) A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation 87: 1571-1578.
- Ricardo JR, Cristovam PC, Filho PA, Farias CC, de Araujo AL, et al. (2013) Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea 32: 221-228.
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, et al. (1997) Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349: 990-993.
- Nakamura T, Endo K, Cooper LJ, Fullwood NJ, Tanifuji N, et al. (2003) The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci 44: 106-116.
- Homma R, Yoshikawa H, Takeno M, Kurokawa MS, Masuda C, et al. (2004) Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest Ophthalmol Vis Sci 45: 4320-4326.
- Tanioka H, Kawasaki S, Yamasaki K, Ang LP, Koizumi N, et al. (2006) Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Invest Ophthalmol Vis Sci 47: 3820-3827.
- Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, et al. (2006) Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells 24: 315-321.
- Yang X, Qu L, Wang X, Zhao M, Li W, et al. (2007) Plasticity of epidermal adult stem cells derived from adult goat ear skin. Mol Reprod Dev 74: 386-396.
- Monteiro BG, Serafim RC, Melo GB, Silva MC, Lizier NF, et al. (2009) Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif 42: 587-594.
- Meyer-Blazejewska EA, Call MK, Yamanaka O, Liu HS, Schlotzer-Schrehardt U, et al. (2011) From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 29: 57-66.
- Reza HM, Ng BY, Gimeno FL, Phan TT, Ang LP (2011) Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev 7: 935-947.
- Lin KJ, Loi MX, Lien GS, Cheng CF, Pao HY, et al. (2013) Topical administration of orbital fat-derived stem cells promotes corneal tissue regeneration. Stem Cell Res Ther 4: 72.
- de Rötth A (1940) Plastic repair of conjunctival defects with fetal membrane. Arch Ophthalmol 23: 522-525.
- Kenyon KR, Tseng SC (1989) Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96: 709-722.
- Turgeon PW, Nauheim RC, Roat MI, Stopak SS, Thoft RA (1990) Indications for keratoepithelioplasty. Arch Ophthalmol 108: 233-236.
- Reinhard T, Sundmacher R, Spelsberg H, Althaus C (1999) Homologous penetrating central limbo-keratoplasty (HPCLK) in bilateral limbal stem cell insufficiency. Acta Ophthalmol Scand 77: 663-667.
- Sangwan VS, Basu S, MacNeil S, Balasubramanian D (2012) Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol 96: 931-934.
- Koizumi N, Inatomi T, Quantock AJ, Fullwood NJ, Dota A, et al. (2000) Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 19: 65-71.
- Dua HS, Gomes JAP, King AJ, Maharajan VS (2004) The amniotic membrane in ophthalmology. Surv Ophthalmol 49: 51-77.
- Fernandes M, Sridhar MS, Sangwan VS, Rao GN (2005) Amniotic membrane transplantation for ocular surface reconstruction. Cornea 24: 643-653.
- Pires RT, Chokshi A, Tseng SC (2000) Amniotic membrane transplantation or conjunctival limbal autograft for limbal stem cell deficiency induced by 5-fluorouracil in glaucoma surgeries. Cornea 19: 284-287.
- Liang L, Li W, Ling S, Sheha H, Qiu W, et al. (2009) Amniotic membrane extraction solution for ocular chemical burns. Clin Experiment Ophthalmol 37: 855-863.
- Kheirkhah A, Raju VK, Tseng SC (2008) Minimal conjunctival limbal autograft for total limbal stem cell deficiency. Cornea 27: 730-733.
- Holland EJ (1996) Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc 94: 677-743.
- Wylegala E, Dobrowolski D, Tarnawska D, Janiszewska D, Gabryel B, et al. (2008) Limbal stem cells transplantation in the reconstruction of the ocular surface: 6 years experience. Eur J Ophthalmol 18: 886-890.
- Ozdemir O, Tekeli O, Ornek K, Arslanpence A, Yalcindag NF (2004) Limbal autograft and allograft transplantations in patients with corneal burns. Eye (Lond) 18: 241-248.
- Reinhard T, Sundmacher R, Spelsberg H, Althaus C (1999) Homologous penetrating central limbo-keratoplasty (hpclk) in bilateral limbal stem cell insufficiency. Acta Ophthalmol Scand 77: 663-667.
- Biber JM, Skeens HM, Neff KD, Holland EJ (2011) The cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea 30: 765-771.
- Shandadfar A, Haug K, Pathak M, Drolsum L, Olstad OK, et al. (2012) Ex vivo expanded autologous limbal epithelial cells on amniotic membrane using a culture medium with human serum as single supplement. Exp Eye Res 97: 1-9.
- Pathak M, Cholidis S, Haug K, Shahdadfar A, Moe MC, et al. (2013) Clinical transplantation of ex vivo expanded autologous limbal epithelial cells using a culture medium with human serum as single supplement: a retrospective case series. Acta Ophthalmol 91: 769-775.
- Lorenzo Y, Berg KH, Ustgaard-Andersen K, Johnsen EO, Ringvold A, et al. (2013) Trypsin for Dissociation of Limbal Cells for Engineering of Grafts May Induce DNA Strand Breaks in the Harvested Cells. J Ocular Biol 1: 6.