Journal of Ocular Biology
Download PDF
Editorial
*Address for Correspondence: Nabil M. Jabbour, M.D., F.A.C.S., Professor, Chief & Fellowship Program Director, Vitreous & Retina Service, WVU Eye Institute, P.O. Box 9193, Morgantown, WV 26506-9193, USA, E-mail: nnjabbour@gmail.com
Citation: Jabbour NM. Protocols versus Algorithms One Factor Hindering Progress in Chemotherapy. J Ocular Biol. 2013;1(1): 2.
Copyright © 2013 Jabbour NM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 1, Issue: 1
Submission: 19 June 2013 | Accepted: 24 June 2013 | Published: 26 June 2013
Protocols versus Algorithms One Factor Hindering Progress in Chemotherapy
Nabil M. Jabbour*
- Vitreous & Retina Service, WVU Eye Institute, President, For Sight Foundation, Morgantown, WV, USA
*Address for Correspondence: Nabil M. Jabbour, M.D., F.A.C.S., Professor, Chief & Fellowship Program Director, Vitreous & Retina Service, WVU Eye Institute, P.O. Box 9193, Morgantown, WV 26506-9193, USA, E-mail: nnjabbour@gmail.com
Citation: Jabbour NM. Protocols versus Algorithms One Factor Hindering Progress in Chemotherapy. J Ocular Biol. 2013;1(1): 2.
Copyright © 2013 Jabbour NM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Ocular Biology | ISSN: 2334-2838 | Volume: 1, Issue: 1
Submission: 19 June 2013 | Accepted: 24 June 2013 | Published: 26 June 2013
Introduction
Cancer treatment has made giant leaps in the past few decades, but most experts feel that even with more research and development we are barely scratching the surface.Having lost both my parents to cancer at a relatively early age, I am very sensitive to the topic of cancer treatment. As a physician who was rarely involved with cancer treatment, I have watched the progress made in the past 30 years – sometimes with wonder; other times with bewilderment. When it comes to non-surgical treatment, especially chemotherapy, it had been difficult for me to see the vast range of efficacy and safety of the same protocols as they were applied to individual patients. The short answer to the big “why?” has always been “people are different and they will respond differently!” This answer was sufficient for me until I started getting involved in a different kind of chemotherapy: pharmacologic treatments of retinal diseases, especially choroidal neovascularization and macular edema.
As with cancer, all the treatment agents were developed and tested in big multi-center studies based on pre-set protocols, AS THEY SHOULD BE. Then those same protocols (just as with cancer treatment) were projected into daily clinical application for treating individual (different) patients with similar but uniquely different lesions. This is a cookie-cutter approach: AN APPROACH THAT SHOULD NOT HAVE BEEN EMPLOYED!
A great deal of good has come from “cookie-cutter” cancer and retina chemotherapy. Life and vision have been extended and improved for many, which is a great human triumph! But, tell that to patients (and their families) who did not respond to treatment. Many would say that it is a fact of life that with any human effort there will always be failures. TRUE, but shouldn’t we make every effort to progressively and continually try to minimize the number of failures? To that end, many advocate that more money and research will continue to improve our outcomes. I used to subscribe to this camp wholeheartedly, but based on my retina experience, I think we are missing a big logical part of the argument. While more research and money has and will continue to result in improvements, the efficiency of our systems needs to be re-examined.
Let us compare our research and clinical application systems to an old car engine with efficiency problems. Would the car get us to our destination faster if a) we pumped more gas into it, or b) we fixed it first?
While our chemotherapy “car” may have several things wrong with its “engine,” I will propose a fix for only one problem that I believe could make a big difference.
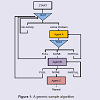
My proposal is based on the premise that our daily clinical application should not be based on “cookie cutter” protocols mirroring the multi-center studies, but rather on a mandatory phase 5 called “the clinical bridge” algorithm. Please see below for details.
The Problem
Wholesale protocols are all based on the following (simplified) model: a certain disease may have no acceptable treatment or several current treatments with or without a “leading” agent. A new treatment agent is developed and is tested for safety then efficacy and/or noninferiority in the presence of a treatment of choice (or “standard of care”).Once statistical significance has been established and the FDA releases the drug, it is recommended that it be used in accordance with the study protocol. To illustrate how this results in a cluster of problems that we in the medical community have been oblivious to, let me use an example I am familiar with: pharmaceutical treatment for CNV. To be fair to all three agents, I will not use a name but rather agent “X”.
The original approved protocol for agent “X” recommended repeated intravitreal injections at 4 or 6 week intervals. After a while, other protocols were developed that recommended 6 or more “loading” doses (in the same frequency as the initial protocol) followed by treatment “PRN” (which can result in various approaches; some protocols recommended injections at certain longer intervals [“treat and extend”]).
Every time an injection is given there are only 3 ways the lesion can respond:
1. Full (or nearly full) response.
2. No (or nearly no) response.
3. Fair to good (partial) response.
Most studies show that with very good agents, of all treated:
Approximately 10-15% of treated lesions fell into Group 1
Approximately 10-15% of treated lesions fell into Group 2
Approximately 70-80% of treated lesions fell into Group 3
Now, let us follow the model of the “wholesale” approach of predetermined regularly spaced injections regardless of the response pattern. Whether we were using monthly, bi-monthly or 12, 6 or 3 “loading doses” etc., the effect is the same, albeit with varying degrees of impact.
Group 3 in general will benefit from the repeated treatment (and as such, drive up the results in favor of the protocol, especially since they are the majority of cases). This effect makes us oblivious to the following set of problems:
1. Group 1 (the responders) may be getting UNNECESSARY and potentially HARMFUL treatment (especially in the long-range – see below). Yet, since they are doing well in the shortterm, they don’t drive the results down.
2. Group 2 (the non-responders) will continue to get worse (on treatment), but they don’t affect the means much because they are a small percentage of the group (and they were on the lower end of results anyway, with little more to lose).
3. Even more disturbing would be eyes in group 3 (the partial responders) which, on repeated treatment, either continue to respond partially, dry up completely (like group 1) or stop responding (like group 2); but because there are not high numbers of these latter two subgroups, they don’t change the means by much. Yet, those particular eyes are being potentially harmed by additional pre-determined injections (just like was mentioned above for groups 1 & 2).
Looking at this analysis objectively makes it easy to see why such a protocol is the correct model to evaluate an agent for approval, but NOT for everyday clinical application where the results are not evaluated as “means” but as individual successes and failures. In everyday clinical application, the non-responders, or those who stop responding later, don’t care if the majority of other patients are doing well. All they know is that they are getting more and more injections while they keep losing vision!
That is why many in group 2 are patients who either give up or jump from one specialist to another, only to get the same result and then give up. They are the ones we call “lost to follow up” when we should simply call them “lost!” Even some in group 1 become “lost to follow up” when they realize they are doing well and wonder why they should continue going to the doctor to “get more shots”. The problem is that they don’t return until their vision worsens (which happens awhile after they have anatomic signs of recurrence & their visual loss could be permanent). Also, remember that those patients in group 3, especially those who become more like group 1 or 2, will behave similarly. All these problems are very serious for individual patients and alone are very important to address, especially when we realize that approximately 35% of all our patients will fall into this category. However, there are other stake holders in this disastrous outcome. Socially and financially, the “lost” patients cost society more emotional and financial burdens with less productivity, compound illness and the need to treat more complex cases. Even the pharmaceutical companies that have a financial interest in selling more drugs will sell less as we “lose” more patients either due to “noncompliance” or “loss of vision beyond treatment” (this is similar to “death” in cancer treatment). So, how can we make this situation better?
The Solution
Custom Designed Approach for Treatment with Algorithms (C DATA)Instead of applying the multi-center protocol to everyday clinical situations, we need to design a “bridge” study with an algorithm model (see Figure 1) that customizes treatment based on individual response instead of a wholesale approach. This will be custom-designed, datadriven instead of cookie-cutter, protocol mandated!
So, if we use the above example and determine the treatment approach for all 3 groups of possible responses right after the first injection, the algorithm will require that:1. Patients (eyes) in group 1 do not get retreatment, but are seen at the same intervals that they would have been treated, and are re-tested. At the earliest sign of recurrence, they would get treated with the same agent again.2. Patients (eyes) in group 2 should be switched to a different treatment (for “rescue”).3. Patients (eyes) in group 3 receive additional treatment of the same agent and get tested at the same period (instead of pre-set treatment) and either get more treatment (if they still have residual activity – still group 3) or follow the protocol for group 1 or 2 if they fall into those patterns later.
Conclusion
This approach guarantees NO UNNECCESSARY TREATMENT and IMMEDIATE RESCUE.With this approach, the patients:
1. Are doing better and are more likely to be compliant.
2. Have less chance to become non-responders.
3. Are less of a burden to society.
In addition, the drug companies may even sell more drugs overall, but these are spread more evenly over time and patients, and the additional cost to society is more than balanced by a healthier, more productive citizenship with fewer emotional, physical and financial demands.
This is but one thing we can do to change a “good” thing (chemotherapy treatment) to a “very good” thing. Other improvements should also be sought. For example, when there is more than one treatment option available, how could we predetermine “nonresponders” and prevent investing time, money, emotional stress and the need for a “rescue”? Such a fix could save precious (otherwise wasted and expensive) time, and accordingly and more importantly, save precious sight and/or lives.