Journal of Oral Biology
Download PDF
Research Article
Secretion of VEGF, TGF-B1 and IGF-1 by Dental- Derived Stem Cells under Hypoxic Conditions
Ariffin F1,3*, Cooper PR1,2 and Scheven BA1
1The School of Dentistry, University of Birmingham, 5 Mill Pool
Way, Edgbaston, Birmingham, B5 7EG, United Kingdom
2Current address: Department of Oral Sciences, Faculty of Dentistry,
University of Otago, PO Box 56, Dunedin 9054, New Zealand
3Current address, Centre of Periodontology Studies, Faculty of
Dentistry, Universiti Teknologi MARA, Sungai Buloh Campus,
47000 Selangor, Malaysia
*Address for Correspondence: Ariffin F, Faculty of Dentistry, Universiti
Teknologi MARA, Sungai Buloh Campus, 47000 Selangor, Malaysia; E-mail:
drfarha@uitm.edu.my
Submission: 26-April-2022
Accepted: 31-May-2022
Published: 03-June-2022
Copyright: © 2022 Ariffin F, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Dental-derived stem cells (DSC) are important cells in tissue regeneration
following tissue destruction. One of the environmental conditions in the
injured tissue is reduce in oxygen level (hypoxia) but the effect of hypoxia on
the DSC is not fully elucidated.
Objectives: This study aims to evaluate the effect of hypoxia on growth
factor production and expression of dental-derived stem cells.
Methods: Rat periodontal ligament stem cells (PDLSCs) and dental
pulp stem cells (DPSCs) were cultured in serum-free media for two or three
days. When the cells achieved 70% confluence, they were incubated under
normoxia (21%) or hypoxia (2%) conditions, before the conditioned media
(CM) that contained the cells’ secretomes were collected and compared with
bone marrow stem cells (BMSCs).ELISA kits were used to analyze VEGF,
TGF-β1 and IGF-1 levels in the collected CM. The reverse transcriptasepolymerase
chain reaction (RT-PCR) was then used to determine the gene
expression of the growth factors.
Results: Hypoxia incubation increased growth factor secretion by the
dental-derived stem cells, and these findings were also supported by the
gene expression analysis of VEGF andTGF-β1. Interestingly, IGF-1 was only
detected in PDLSC CM, and these data were supported by prominent IGF-I
gene expression and an inverse relationship with IGF-BP1 expression by
PDLSC, compared with DPSCs and BMSCs. TGF-β1 secretion by BMSCs
was not influenced by hypoxic incubation.
Conclusion: Hypoxic incubation of the dental-derived stem cells alters
growth factor content in the secretomes, and IGF-1 was only detected in the
PDLSC secretome
Keywords
Dental-derived; Stem cells; Secretome; Growth factors; Hypoxia
Introduction
Periodontal tissue regeneration is a complex process involving
the periodontal ligaments (PDL) and other complex structures,
such as alveolar bone and cementum, which are usually diminished
during periodontitis. In recent years, more research has been moving
towards stem cells application in periodontal regeneration [1].
Mesenchymal stem cells (MSCs) are undifferentiated multipotent
cells that can differentiate into mesoderm cell lineages, including
osteogenic, adipogenic and chondrogenic lines. MSCs are widely
distributed throughout the body, such as in the bone marrow stem
cells (BMSC) and dental-derived stem cells [2], which include dental
pulp-derived stem cells (DPSCs) and periodontal ligament stem
cells (PDLSCs) [3,4]. However, most clinical trials using MSCs in
humans are in the early stages [5], and very few are related to dental
regenerative therapy [6-9].
Currently, there are several limitations in the clinical application
of MSCs, including the possibility of ectopic tissue formation [10], injected MSCs being short-lived or removed by the circulation and
clearance by the liver and lung [10]. The secretome is defined as cell
secretions containing multiple growth factors, cytokines, enzymes,
exosomes, micro RNA and other soluble mediators [14]. Many
researchers have studied the bioactive molecules in MSC secretomes
[15-19]. For instance, secretomes of mesenchymal and dental stem
cells have been tested for tissue regeneration in vitro and in vivo, such
as nerve and bone regeneration [14,15,,20,21].
Despite the numerous attempts to identify the MSC secretome
components, the exact content and factors contributing to their
differential effects, including different oxygen concentrations during
tissue culture remain inconclusive. Interestingly, disease-relevant
conditions such as hypoxia have been shown to induce stem cells to
increase growth factor expressions like VEGF, TGF-β1 and IGF-1,
which are essential for cell survival and tissue regeneration [18,22-24]. Consequently, it has been concluded that stressed cells produce
more protective secretomes to create an improved environment
for cell survival. Furthermore, cell metabolic products may decline
under serum deprivation conditions, resulting in a less cytotoxic
environment [16]. However, to date, dental-derived stem cells
studies on hypoxic incubation for secretome production are still
limited [24-27]. Thus, this study aimed to evaluate VEGF, TGF-β1
and IGF-1 levels released in conditioned media from PDLSCs and
DPSCs secretome compared with the more widely studied BMSCs
secretome, particularly in hypoxic conditions.
Materials & Methods
Cell Cultures
MSCs were isolated from six-week-old Wistar-Han rats
(Pharmaceutical Sciences Animal House, Aston University,
Birmingham, UK) with an average weight of 120g. BMSCs were
isolated from rat femurs, PDLSCs from the PDL tissue surrounding
the roots of molar teeth and DPSCs were obtained from pulp tissue
of incisor teeth, as previously described by [28,29]. The isolated
cell populations were initially cultured at 37oC with 5% carbon dioxide (CO2)(RS Biotech) in alpha minimum essential medium
(α-MEM) (Biosera, UK) containing 10% Fetal Bovine Serum (FBS)
(Gibco) and1% penicillin/streptomycin/amphotericin (100 units/mL
penicillin with 100 μg/mL streptomycin and 2.5 μg/mL amphotericin)
(Sigma-Aldrich, UK). The multi-potentiality of the cultures, including
osteogenic and adipogenic differentiation, were verified based on
previous studies and were validated in the laboratories [29].
Polymerase Chain Reaction (PCR) Analysis
Stem cell markers expression were analysed using semiquantitative
PCR [30]. RNA was isolated using the RNeasy kit (Qiagen,
UK), and cDNA synthesis was generated using the TetroTM cDNA
Synthesis Kit (Bioline,UK). The primers used for gene expression
analysis are listed in Table 1. The specific gene band intensity was
normalised to the GAPDH band intensity, and comparisons were
made between the three cell types.
MSC growth in Hypoxia
The MSCs from passages three to five were seeded into 35mm
dishes with a cell density of 2.5 x 104 cells/ml to evaluate the cell
numbers and growth. The cells were either cultured in “normal”21%
oxygen incubation or were incubated under hypoxia conditions (2%
oxygen). The culture media in each well was refreshed every three
days. In addition, the cells were cultured in serum-free media.
Viable cell counts were performed to monitor cell growth. The
cell suspension was mixed with Trypan blue cell stain in a micro
centrifuge tube and incubated for 10 min at room temperature to
allow dye uptake by the cells. The viable and non-viable cells were
counted manually under a microscope (Zeiss, Germany). Five counts
per sample were recorded, and an average value was calculated. The
cell count was repeated every three days until day 12. Experiments
were performed in quadruplicate.
CM Collection
The BMSCs, PDLSCs and DPSCS of passage three to five were
cultured with 10% FBS culture media until 70-80% confluent.
Cultures were washed three times with 3ml PBS before 15 ml serumfree
media were added into each flask. The cells were incubated in
either a standard incubator with 21% oxygen or in a hypoxic incubator
(Galaxy 48R, New Brunswick), in which the oxygen concentration
was set to 2%. CM was collected on the second and third days of
culture, filtered with a 0.2μm membrane filter (Sigma-Aldrich) and
stored at -200C until ELISA analysis was performed. The experiment
was conducted in triplicates.
Growth Factor Analysis
The VEGF, TGF-B1 and IGF-1 levels in each CM were determined
using commercially available rat ELISA kits (R&D Systems) according
to the manufacturer’s guidelines.
Statistical analysis
The data obtained in this study were analysed using SPSS Version
22 for Windows. Independent sample t-test was used for experiments
involving two groups, while One-way ANOVA for analysis of more
than two groups, along with Bonferroni test as a post-hoc analysis.
The findings were statistically significant at p <0.05.
Results
The BMSCs, PDLSCs and DPSCs used in this study demonstrated
typical MSC characteristics such as multi lineage differentiation and
stem cell-related markers expression, including CD105, CD29, CD44
and CD90 as shown in the qRT-PCR analysis [30]. Furthermore,
c-myc was expressed, which is considered a stem cell-related gene
for cellular metabolism and proliferation. The expression of CD105,
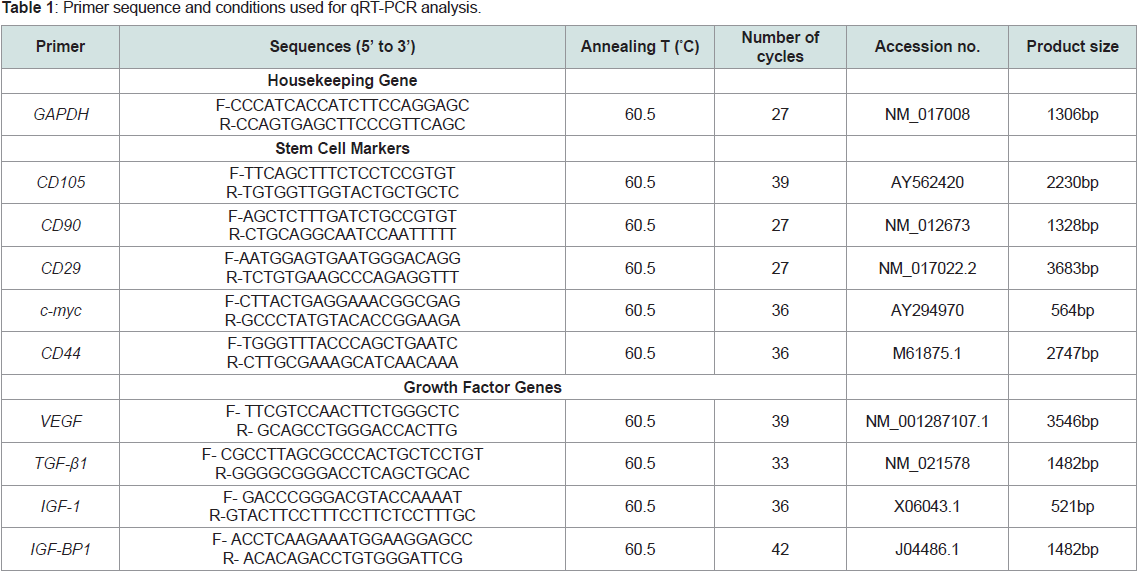
CD90 and CD29 in PDLSCs and DPSCs were significantly lower
than BMSCs, however CD 29, CD44 and c-myc expression appeared
similar across all MSCs (Figure 1).
Figure 1: Stem Cell Markers. Stem Cell Marker Analysis. Semi-quantitative PCR analysis of gene expression of CD105, CD90, CD29, CD44 and c-mycin
BMSCs, PDLSCs and DPSCs. Y-axis is the relative expression to GAPDH. Statistical comparison was performed using BMSCs as control with *p-value<0.05
and **p-value<0.001, n=3.
MSC growth:
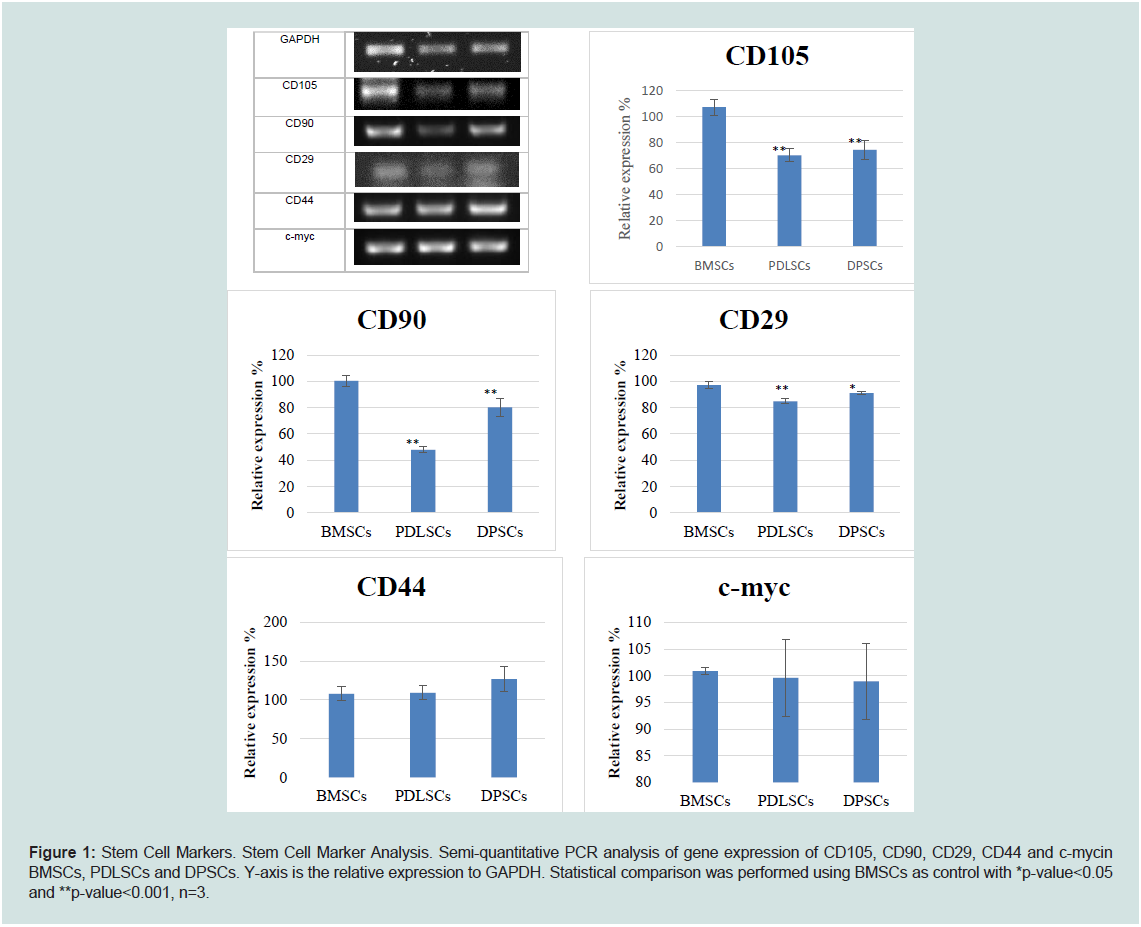
Hypoxic conditions
The DPSC cultures exhibited a significant increase in cell numbers
under hypoxic conditions. On the other hand, the PDLSC growth rate
increased significantly in the hypoxic environment until day 6 but
decreased at day 9 (Figure 2). In contrast, the BMSC cultures had
significantly increased cell numbers under normoxic compared to
hypoxic conditions on day 12 (p-value<0.001).
Figure 2: MSC growth under normoxic and hypoxic conditions. Viable cell count of the cells in each incubation environment was analysed and statistically
compared using normoxic cultured cells as control with *p -value<0.05 and **p-value<0.001. Mean cell count +/- SD (n=4).
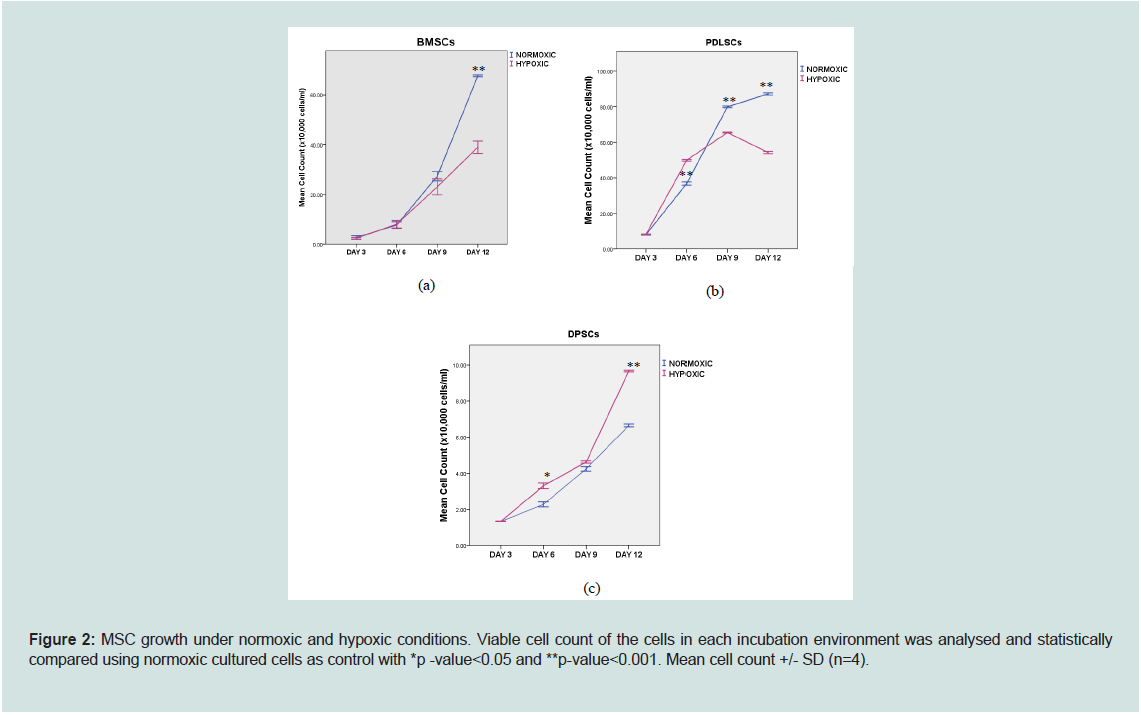
Serum-free media culture conditions
MSCs cultured in serum-free media for three days showed no
differences in cell viability and numbers under different oxygen
incubations except for PDLSCs, which had significantly reduced
cell numbers in hypoxia incubation. Additionally, no significant
differences were recorded for viable cell numbers between BMSCs
and DPSCs incubated in normoxia or hypoxia at three-time intervals,
except the ones shown in Figure 3a and c. PDLSCs incubated in both
normoxia and hypoxia incubations showed increased cell numbers at
all time points, and the increases were statistically significant (Figure 3b). Generally, the result showed that cell viability was maintained in
this study.
Figure 3: MSC number in serum-free media and different oxygen concentration. Data show mean cell numbers for the three cell types. The statistical analysis
was performed between 1) between normoxic and hypoxic cells at all time points, and 2) between day 1 and day 2 or day 3. (n=5).
____________Comparison between normoxic cells
--------------------Comparison between hypoxic cells
Levels of growth factors secreted in serum-free media cultures
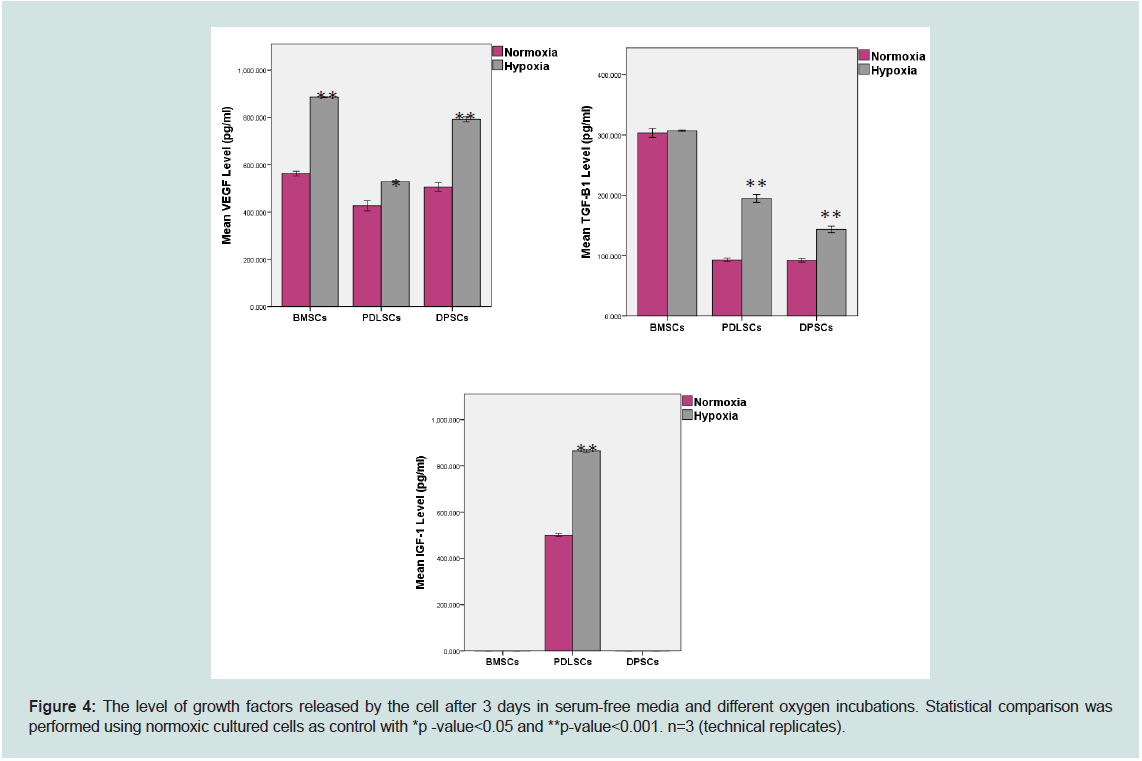
Hypoxic-incubated BMSCs, PDLSCs and DPSCs secreted
significantly greater VEGF than cells in normoxic conditions (Figure 4). Interestingly, IGF-1 was only detected in PDLSC cultures,
where the hypoxic-incubated cells produced higher IGF-1 than the
normoxic-incubated cells (Figure 4). Moreover, the TGF-β1 level was
statistically higher in hypoxic PDLSCs and DPSCs.
Figure 4: The level of growth factors released by the cell after 3 days in serum-free media and different oxygen incubations. Statistical comparison was
performed using normoxic cultured cells as control with *p -value<0.05 and **p-value<0.001. n=3 (technical replicates).
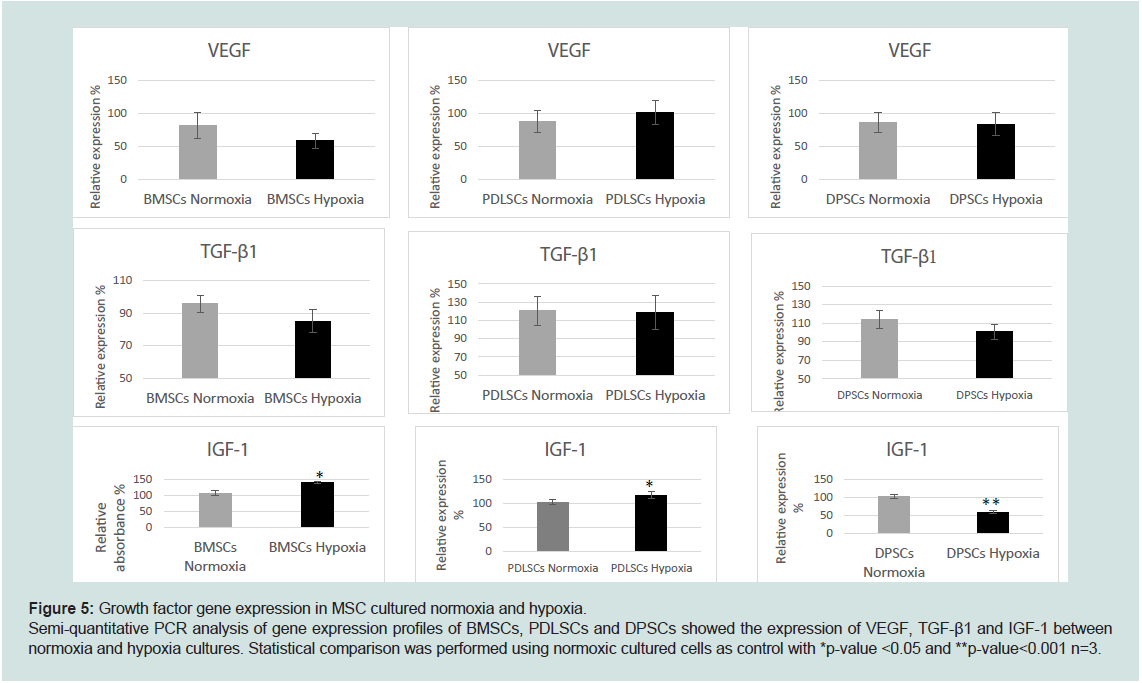
These findings were subsequently corroborated by RT-PCR gene
expression analysis. The differences in VEGF and TGF-β1 expression
in all three cell types were not significant, although VEGF expression
by BMSCs normoxia samples was higher than the hypoxia samples.
BMSC and PDLSC hypoxic cultures expressed higher IGF-1, in
contrast normoxic DPSCs expressed higher IGF-1(Figure 5).
Figure 5: Growth factor gene expression in MSC cultured normoxia and hypoxia.
Semi-quantitative PCR analysis of gene expression profiles of BMSCs, PDLSCs and DPSCs showed the expression of VEGF, TGF-β1 and IGF-1 between
normoxia and hypoxia cultures. Statistical comparison was performed using normoxic cultured cells as control with *p-value <0.05 and **p-value<0.001 n=3.
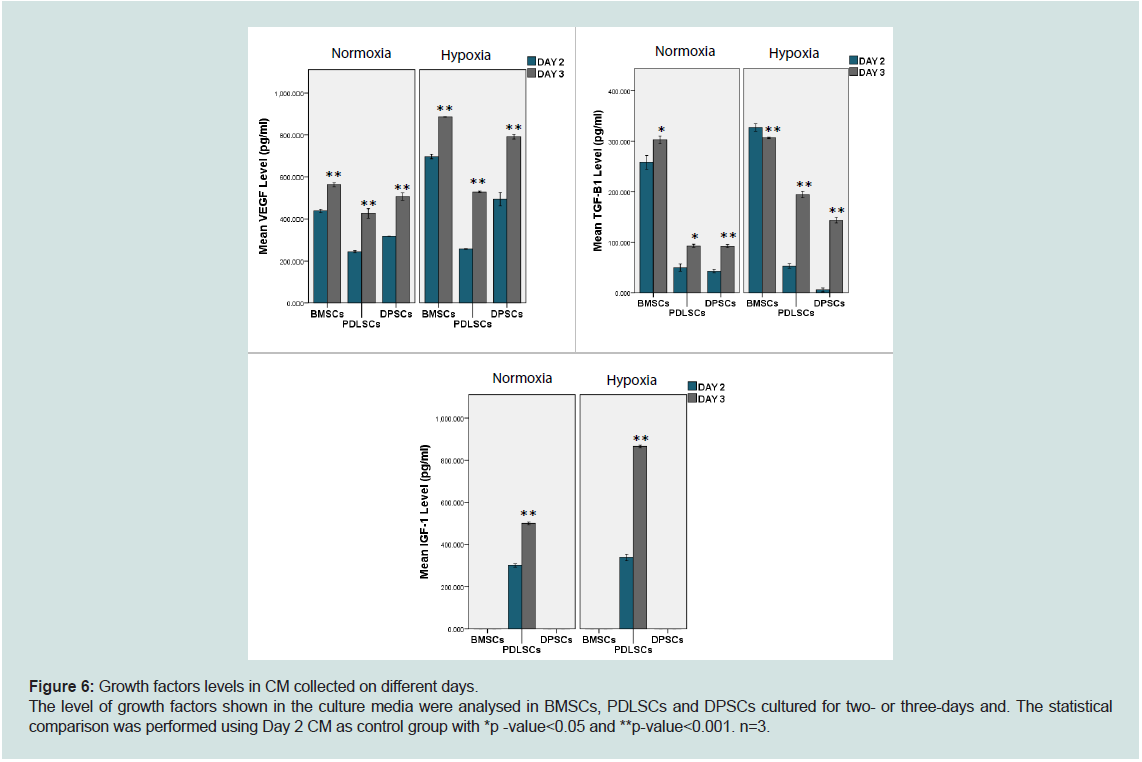
All samples from the different cell types under different
incubation conditions demonstrated higher VEGF, TGF-β1 and
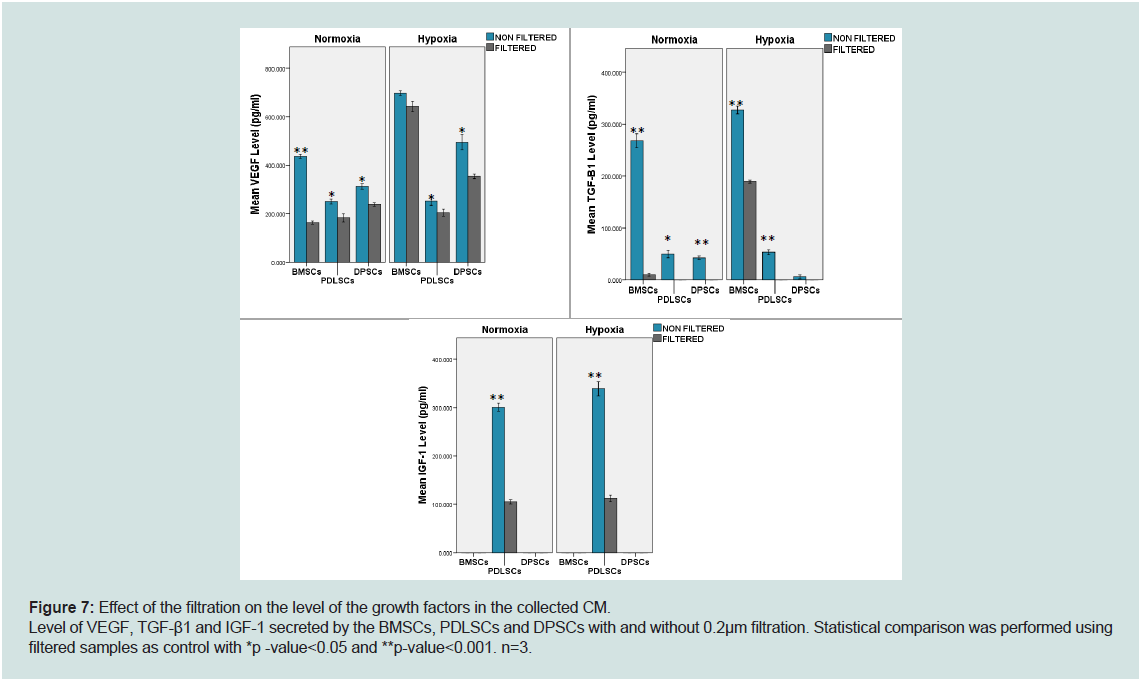
IGF-1 levels on day 3 compared today 2, except for TGF-β1 secreted by BMSCs (Figure 6). Apart from that, the filtrated CM contained
significantly lower growth factors except for VEGF in BMSCs under
hypoxic conditions (p-value =0.81, Figure 7) and TGF-β1 in DPSCs
under hypoxic conditions(p-value = 0.215, Figure 7).
Figure 6: Growth factors levels in CM collected on different days.
The level of growth factors shown in the culture media were analysed in BMSCs, PDLSCs and DPSCs cultured for two- or three-days and. The statistical
comparison was performed using Day 2 CM as control group with *p -value<0.05 and **p-value<0.001. n=3.
Figure 7: Effect of the filtration on the level of the growth factors in the collected CM.
Level of VEGF, TGF-β1 and IGF-1 secreted by the BMSCs, PDLSCs and DPSCs with and without 0.2μm filtration. Statistical comparison was performed using
filtered samples as control with *p -value<0.05 and **p-value<0.001. n=3.
Discussion
The hypoxia-stimulated proliferation of MSCs has been reported
in previous studies [31,32]. A low oxygen environment has been
proposed to maintain their stemness by preserving the undifferentiated
state of the cells. Conversely, increased oxygen concentration can
promote MSC differentiation [31]. Similar findings were recorded for
dental-derived stem cells in the current study. The PDLSC numbers
increased in a hypoxic environment at the onset of the culture period;
however, cell growth decreased by day 9. Progenitor cells residing in
the periodontal ligament are speculated to have a reduced oxygen
environment, as demonstrated by a previous in vivo study of tooth
root development [33]. The increased DPSC numbers under hypoxia
conditions are consistent with other studies that utilised animal and
human samples [34,35].
When the cells were cultured in serum-free media for up to
three days, there was a decrease in BMSC cell viability after two days
and after one day culture for DPSCs, under normoxia and hypoxia
conditions. In contrast, PDLSCs were constantly viable, which may
be contributed by active IGF-1as a survival-promoting and antiapoptotic
factor in the culture. Previous studies have reported that
the epigenetic programming of the IGF-1 gene in MSCs may occur when cultured in serum-deprived conditions, and IGF-1-depleted
CM demonstrated higher cell apoptosis compared with cells cultured
in non-depleted IGF-1 CM [36]. Furthermore, IGF-1 is one of the
growth factors involved in cell metabolism and regulates oxidative
stress resistance [37]. Thus, this finding may be related to the demand
for PDLSCs as they exhibit the fastest turnover rate in the body [38].
Similarly, the present study showed an increase in IGF-1 level by
PDLSCs in hypoxic incubation.
IGF-1was exclusively expressed by PDLSC, while IGF-BP1 was
present at relatively low levels in PDLSCs compared with BMSCs
and DPSCs. This finding may be related to PDLSCs relatively high
turnover rate and the metabolically active state of PDL cells [38,39].
Consequently, the presence and role of IGF-I and its multiple binding
proteins in different MSCs require further investigation. Notably, a
recent microarray study demonstrated relatively high levels of IGFBP-
6, -2 and -4, but lower IGF-BP1 and -3 in the secretome of human
PDLSCs [40]. In addition, a higher level of IGF-2 in PDLSC CM was
reported compared to IGF-1 [40].
The relatively low IGF-1 gene expression in the DPSC samples
aligned with previous reports. An increase in IGF-BP2 and IGFBP3
gene expression was observed, although there was no mention
of IGF-BP1 expression [41]. Furthermore, the low level of IGF-1
concomitant with the high level of IGF-BP1 in BMSC may be related
to the role of BP in protecting IGF-1 degradation within the bone
marrow niche [42]. Apart from IGF-BP1, other IGF-BPs were also identified in CMs from BMSCs, including IGF-BP-2, -3, -4 and -6 in
their microarray profiles [17,23]. However, the specific roles of this
IGF-BPs within this context have not been fully understood.
Hypoxia incubation can alter VEGF secretion. In this study, VEGF
production was higher in hypoxia conditions for all three cell types.
Nonetheless, this outcome was contradictory at the transcriptome
levels since there were no significant differences between normoxia
and hypoxia conditions for all cell types. This observation may be the
result of translational differences and cellular storage of VEGF [43].
VEGF was highly secreted by BMSCs inhypoxia after three days
of culture, whereas PDLSC CM demonstrated the lowest VEGF
levels, although hypoxia incubation increased its production. The
effect of hypoxia incubation on VEGF production corroborated with
other studies. For instance, VEGF production was higher by PDLSCs
and DPSCs incubated in hypoxia conditions with 1-2% oxygen
concentration [34]. The relatively high production of VEGF by BMSCs
compared with DPSCs and PDLSCs could reflect the significance of
cell origin from the bone marrow. This tissue is closely associated
with the regulation of angiogenesis and provides a known source for
endothelial progenitor cells, not only under normal physiology but in
any pathological conditions in the body [44].
TGF-β1 is a critical growth factor in tissue repair and regulates
cell differentiation [45]. In this study, TGF-β1 was highly expressed
by BMSCs compared to PDLSCs and DPSCs, and hypoxic incubation
induced both cell types to produce more TGF-β1. Nevertheless, there
was no difference in TGF-β1 production by BMSC under different
oxygen concentrations, indicating that oxygen is not essential for
TGF-β1 production by BMSCs.
There are various methods available for CM collection to study
the secretome from cultured cells. Most studies have opted for 48 h
[14,17,19,20,24], while the others collected the CM on the third day
or after. The CM was collected after two days to obtain richer growth
factors or bioactive molecules, but the media could be a limiting
factor because of the cells metabolic activity [16]. One study collected
the CM of rat DPSCs, BMSCs and ADSCs after three days for mass
spectrometry analysis and reported various proteomic profiles
associated with MSC secretome and angiogenesis, cell migration and
inflammatory response [46]. Another study reported the collection
of CM from DPSC cultures on every 4th day up to 24 days culture.
Notably, it was found that the cell viability was higher at shorter
collection periods [16]. In the present study, CM was collected at
less than four days to reduce the chances of contamination with byproducts
of the cell metabolic activity. The data indicated that a longer
culture time allowed more growth factors to accumulate in the CM.
Another variable in the CM processing technique is filtration, as
reported in the literature [10,16,20,46]. The current study recorded
lower growth factor concentrations in media filtered through a
0.2μm membrane, commonly used for sterile filtration. This finding
suggested that bioactive molecules such as VEGF, TGF-β1 and IGF-1
are entrapped and filtered out.
Important growth factors were detected in BMSCs CM, PDLSCs
CM and DPSCs CM compared to serum-free media. However, the
serum utilised during cell expansion in the laboratory is usually
animal-based, thus, carrying the risk of cross-infection from animals
to other species and eliciting adverse reactions [47]. Furthermore,
clinical studies using stem cell secretomes are limited and only involve the BMSC secretomes [48]. Therefore, since the cells secretome were
retrieved in the form of CM and serum-free in this study, they are
highly applicable for translational research and human clinical
studies. Nevertheless, further investigations are crucial on dosages,
storage, and long-term safety of the CM.
Conclusion
In summary, hypoxia incubation can potentially promote dentalderived
stem cell cultures to generate optimum VEGF, TGF-β1 and
IGF-1 within the secretome. In addition, it can be concluded that the
secretome derived from PDLSCs was notably different compared
to the other two cell types in IGF-1 production. Nevertheless,
further study is required to determine the dental-derived stem cells
secretomes mechanism of action to be translated into therapeutic
application.
Funding
This study was funded by the Ministry of Higher Education,
Malaysia.
Acknowledgement
The authors acknowledge the help and support of the laboratory
technicians in the School of Dentistry, University of Birmingham,
UK.