Journal of Oral Biology
Download PDF
Review Article
The Oral Microbiome: Health Benefits, Disease, and Neurodegeneration
Rozema N1, Schuiling M2,3, Thompson SO4 and Griffin GD2,3*
1Department of Chemistry, Hope College, USA
2Department of Biology, Hope College, USA
3Department of Psychology, Hope College, USA
4US Army Dental Corps, Office of the Surgeon General, USA
*Address for Correspondence: Griffin GD, Department of Biology, Department of Psychology, Hope
College, 35 East 12th Street, Schaap Science Center Room 2019,
Holland, MI 49423, Phone: 616-395-6813, USA;
E-mail: griffing@hope.edu
Submission: 22 June, 2019;
Accepted: 03 August, 2019;
Published: 06 August, 2019
Copyright: © 2019 Rozema N, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
It is a well-known idea that humans have a large set of bacteria
housed inside them, but it was not until the year 2001 that Joshua Lederberg
would officially coin the term ‘microbiome’ in reference to the ecological
community of commensal, symbiotic and pathogenic microorganisms that
share our body space. Since that time, the advancement of technology
has allowed for much more efficient sequencing and studying of the exact
microbiota dwelling symbiotically within humans. The oral cavity houses
the second highest amount of microbiota in the human body, 700 species,
only behind the gut which boasts over 1,000 different bacterial species. This
review article explores the large database of research that supports the
complex relationship humans have with the bacteria inside them. Many
different factors contribute to the formation of a person’s oral microbiome
including a person’s lifestyle and diet choices, as well as societal factors
such as antimicrobial and pesticide use. Research has suggested that
the relationship between microbiome and host is constantly changing to
meet the ever-changing conditions pertaining to human life. While some
bacterial species can be assistive in helping humans develop adaptive
immunity and biofilms, other species can contribute to health complications
such as gingivitis, dental caries, and even neurodegenerative diseases.
While a lot of research is still needed to establish exact mechanisms on how
these bacteria acquire entry into other parts of the body and the central
nervous system, it is clear that their impact expands farther than just their
oral cavity home. Bacteria in conjunction with human life cannot be seen
as all-assistive or all-destroying. Depending on the bacteria present and
its location within the body, its effects can be extremely life-sustaining or
extremely life-threatening.
Keywords
Adaptive immunity; Bacteria; Diet; Microbiota; Microbiome; Oral
cavity; Neurodegenerative disease
Introduction
From a very young age kids are taught that germs are bad. They
are strongly advised to wash their hands for the entire duration of the
ABCs in order to cleanse themselves from any remaining bacteria they
had acquired while playing outside in the mud. The fact of the matter
is that though we may try to rid ourselves of microorganisms, they
are impossible to escape. It is estimated that the human body houses
around 30-50 trillion bacterial cells at any given point [1], a number
that is sure to make any parents of a toddler squirm. In actuality, while
some bacteria are harmful and can foster the spread of pathogens
and neurodegenerative diseases, a large proportion of bacteria dwell
symbiotically within us, helping us perform various bodily functions
that would otherwise not be possible. Most review articles tend to
focus on a single topic concerning the oral microbiome, be that the
benefits, the oral diseases or the neurodegenerative implications
these bacteria have on humans. However, there is an extremely large
overlap in the role that bacteria can play. For instance, Tomás et al. in
2012 discuss one type of bacteria that leads to plaque formation, yet
also hinders the growth of some more dangerous bacteria in the same
area [2]. For this reason, it is extremely difficult to separate the good,
the bad, and the ugly that this microbiota elicit in the human body.
It is the goal of this review article to give the reader a more holistic
understanding of all the roles that these bacteria can play, as well as
to give insight on how our own lives and the lives of said organisms
have become even more connected in terms of immune system and
overall health than was previously believed. With this paper, we seek
to answer the question: what purpose do these microorganisms serve
and are they primarily good or bad? These bacterial populations are
dynamic in nature, adapting daily to new environmental factors and
lifestyle choices that humans make without even thinking about it.
Factors Affecting the Oral Microbiome
The first factor that greatly impacts the oral microbiome is the
dietary choices of humans. Studies have looked at the progression of
bacteria. Over the course of human history there have been two major
shifts in microbiome occupancy [3]. The first shift came during the
transition from a hunter-gatherer lifestyle to an agriculturally based
lifestyle about 10,000 years ago [4]. There is evidence that during
prehistoric times when our ancestors were primarily hunters and
gatherers, there were a lower number of cariogenic bacteria or bacteria
that can cause tooth decay [5]. Despite this, the oral microbiome was
still very diverse accommodating the vast variety of foods that these
hunter-gatherers would eat.
During the onset of the farming lifestyle when consumption of
carbohydrates such as wheat and barley greatly increased, there was a
positively associated increase of these cariogenic bacteria as well [6].
It was at this time that tooth decay and periodontal disease started
to present themselves. These two diseases are both brought about by
the buildup of plaque. Plaque development occurs when a bacterial
biofilm mineralizes with calcium phosphate. After mineralizing the
plaque is deposited around the gingiva or the gums [7]. The second
shift of diet was during the industrial revolution between the years
1760 and 1830. This can most likely be attributed to the newly
developed means of food production including machinery as well as
new preservatives to extend the shelf life of foods [8]. It was at this
point that the once absent cariogenic bacteria actually became the
dominant species resulting in a large decrease in bacterial biodiversity in the oral cavity. Research suggests that this shift in diversity and
prevalence of cariogenic bacteria is one of the primary causes of 92%
of adults having tooth decay in their lives and half of American adults
having chronic periodontal disease [9].
Linked with diet, poor oral hygeine has a profound influence on
the oral microbiome. Common observances noted among patients
with poor oral hygiene include, a high plaque index, generalized
cervical calculus, rampant caries, halitosis, and low salivary flow [10].
These clinical findings are closely tied to long-term acidification.
Without proper care and clinical treatment, these factors cause the
pH of the oral environment to fall below a pH of 5.0. This decrease in
pH causes particular bacteria to dominate the oral microbiome. More
specifically, members of the genus Streptococcus, Capnocytophaga,
Eikenella, Campylobacter, and Actinomyces all increase inviability
in the mouth without proper oral care. These forms of bacteria are
mainly from the yellow, green, and purple complex of subgingival
microbiomes. These groups are early colonizers of the tooth surface,
and their growth typically precedes the predomination of gramnegative
orange and red complexes [11].
The next factor that impacts the oral microbiome is antimicrobial
use. With the exponential growth of the human population we
have needed to just as quickly adapt our food production styles.
One way we have chosen to make this adaption is through the use
of antimicrobials and pesticides. From this increased exposure to
avoparcin and other antibiotics, some bacteria have developed partial
or complete immunities to certain drugs such as vancomycin or
chemicals in human saliva [12]. Because of this immunity, some types
of bacteria housed in the oral microbiome have flourished, increasing
in number. Depending on the bacteria, this can be either a good or
bad thing. One example is the bacteria Staphylococcus aureus and
Enterococcus. These two infectious bacteria have developed resistance
to antimicrobials which is one potential reason that Staph infection
rates have increased significantly in the past decade [13]. European
countries have already taken action to slow the development of
resistance, removing avoparcin from their animal feed in 1997 [14].
Despite the best efforts by governments to slow this process, antibiotic
resistance in oral bacteria seems to be an unavoidable end with new
research indicating that in addition to exposure to pesticides, the
process of aging itself correlates to an increased resistance exhibited
in certain oral bacteria [15].
The final factor that impacts the oral microbiome is lifestyle.
Because of the dynamic nature of the oral microbiome, short term
changes in factors such as body temperature, neurotransmitter
levels, and respiratory rates can account for fluctuations in bacterial
richness. One experiment performed by Kupchak et al. in 2017
investigated the impact that completing a 164-km cycling event had
on the types and quantities of bacteria present [16]. They found that
while there was no significant change in diversity, the abundance of
certain types fluctuated, specifically an increase of Firmicutes and a
decrease in Bacteroidetes. The bacterial phylum Firmicutes have been
extensively studied and it is well documented that individuals with
diabetes tend to have higher abundance of this phylum compared to
other oral microbiota [17]. However, it is undetermined as to whether
this increase of Firmicutes is a potential cause of diabetes or just a byproduct
of the disease. Another key factor relating to lifestyle is oral hygiene. Although extensive work has yet to be performed on this
topic, research like this provides viable evidence that lifestyle plays a
much larger role than we once thought.
Health Benefits of the Natural Oral Microbiome
There can be a negative connotation associated with oral bacteria,
suggesting that the roles they play are all negative. However, there
have been recent ideas that describe humans as a supraorganism
which is made up of both the human body and its microorganisms
[18]. Rather than viewing all these bacteria as parasites feeding off
their human host and causing disease, we should instead consider
some of them as symbiotic maintainers of our homeostatic state. A
few of these benefits of microbiota include immunological priming,
down-regulation of excessive pro-inflammatory responses, regulation
of gastrointestinal and cardiovascular systems, and colonisation by
exogenous microbes [19].
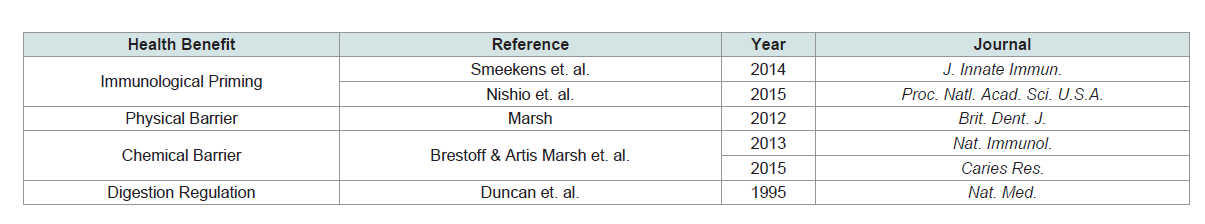
Microbiota play an essential role in the development of adaptive
immunity. The human immune system is under constant exposure to
outside viruses, bacteria, and other pathogens that could cause sickness
and immune responses. Humans and the microbiota in the oral
cavity have evolved together in service for each other. These bacteria,
like any other bacteria that humans come in contact with, have the
potential to elicit an immune response [20]. However, because the
human body has adapted to accommodate this microbiota, no such
response is shown in healthy individuals. This idea is reinforced in a
study where patients with primary immunodeficiencies (lacking in
their well adapted immune system) suffered from infections caused by
common microbiota found in human skin and mucosal microbiome
[21]. Extensive research has been done on the microbiota present in
the gut and its specific effects on immunity. One example discusses
the interaction of bacteria with Th17. These cells are essential in the
production of IL-17 a key part of the proinflammatory response
[22]. The adhesion of specific gut microbes to intestinal epithelial
cells is a cue for the induction of Th17 cells, and thus of the eventual
immune response cascade [23]. Although less extensive work has
been performed pertaining to the oral microbiome, it is plausible to
suggest that similar response pathways can be triggered via bacteria
in the oral cavity, and future studies will undoubtedly bring this idea
to fruition.
The formation of biofilms along the gingiva in the mouth
exemplify the competitive exclusion principle seen in population
biology. This principle states that two species competing for the
same limiting resource cannot coexist at constant population values.
Therefore, biofilms of microbiota are taking up space that other
potential bacterial tenants would need to colonize [24]. For this
reason, the coevolved bacteria act as a force field preventing dangerous
bacteria from taking up residence in our bodies. Additionally, the
native bacteria S. mutans has been found to produce peptides that
inhibit the biofilm formation of Candida albicans, a pathogen that
could potentially cause oral fungal infections [19]. In this way, the
microbiota not only act as a physical barrier, but also a chemical one
(Table 1).
Historically speaking, the commensal bacteria in the oral cavity
were well adapted to the diets of ancient humans, allowing for the
effective digestion of meals. Bacteria in the phylum Ruminococcaceae were associated with good health and were found in high frequency
[5]. Unfortunately, these well adapted bacteria are present in much
lower frequencies in humans today, most likely due to our change in
diet. Research is still being performed to determine current roles that
high frequency bacteria play in digestion, although it is suspected to
be large. The bodies first line of breaking down carbohydrates comes
in the form of salivary amylase. This liquid is made up of digestive
enzymes in addition to a few other chemicals. Recent findings
show that in addition to the body’s natural digestive enzymes,
the commensal bacteria aid in the digestion including extraction,
synthesis and absorption of many nutrients and metabolites [23].
One such case is the reduction of nitrate to nitrite. This is carried out
by anaerobic bacteria through the production of nitrate reductase
enzymes [26]. This nitrite will subsequently play a key role in
cardiovascular health, acting as a strong vasodilator and antimicrobial
agent. More generally, bacteria all along the gastrointestinal tract have
been associated with the production of bile acids which are important
factors in the digestion of fatty acid chains [25]. While research is still
examining more specific roles of modern day oral bacteria, it is likely
that they have continued to co-evolve with us humans as our digestive
needs have shifted.
The Oral Microbiome and Disease
Despite the coevolution of these bacterial species and their
numerous health benefits, modern diets and dental habits have
greatly impacted the oral cavity and an influx of negative bacteria have
claimed the territory as their home. The increased exposure humans
have to heavy metals, disinfectants, and antibiotics have led to the
positive selection of bacteria with resistances to such compounds [27].
While not all these newly adapted bacteria brought an onset of dental
problems, a significant number did such as Streptococcus mutans being able to outcompete other oral bacteria species and becoming one of the leading causes of tooth decay [28]. Oral and periodontal diseases (caries, chronic and aggressive periodontitis, mucositis, and
gingivitis) are associated with changes to the microbiome, specifically prevalence of anaerobic bacteria within the flora [29]. Most diseases
are rarely caused by a single bacterium, but rather a combination of
species or complexes.
Gingivitis simply refers to the inflammation of the gum tissue. It
tends to be acute and fairly manageable. However, left untreated, it can
progress to later stages called periodontitis where the inflammation
reaches the bone and soft tissue associated with anchoring the teeth
resulting in the eventual loss of teeth [30]. The primary cause for such
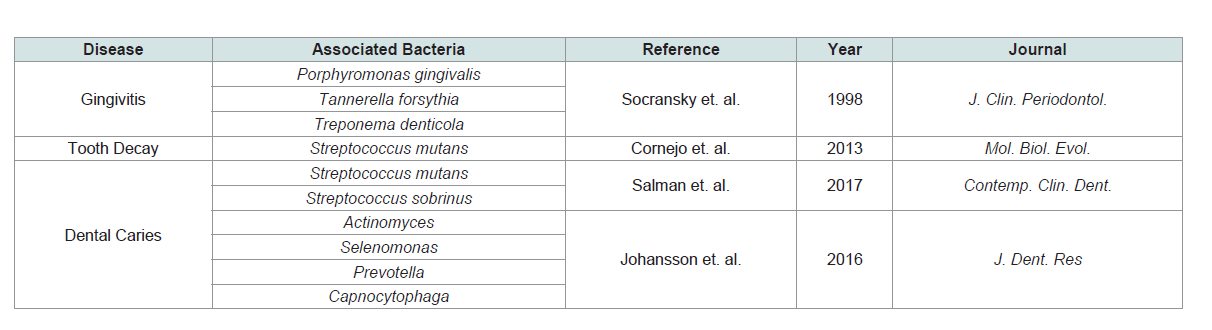
diseases can be traced to a complex of bacteria referred to as the ‘red
complex’. This complex is made up of P. gingivalis, T. forsythia, and
T. denticola [11]. While other bacteria can be linked to these diseases,
it is the red complex that is most often associated with the onset. The
red complex tends to appear in later stages of biofilm development,
suggesting that earlier bacterial species, referred to as keystone
pathogens, will impair the immune response of the host and prepare
a habitat for the red complex to eventually succeed [31]. While the
red complex works as a unit, research has linked high amounts of T.
forsythia with greater severity of lesions or pockets, and T. denticola
with greater severity of bleeding [32] (Table 2).
Dental caries encompasses both moderate and extreme tooth
decay. It is one of the most prevalent human bacterial infections and
similar to periodontitis, can lead to tooth loss. In the past, Streptococcus
mutans has been viewed as the main cause of dental caries; however,
recent research has shown that not all subjects with caries have
detectable levels of this bacteria, proving that there are other bacteria
involved [33]. It is widely accepted that the bacteria responsible
thrive under low pH conditions, since the ingestion of acidic meals
can recruit these bacteria. Interestingly enough, there seems to be a
socioeconomic distinction about what bacteria are responsible in each
individual. In a comparative study between Romania and Sweden it
was found that in Romanians with dental caries where dental health
care is uncommon, seemed to be infected with the classic. S. mutans
and S. sobrinus. Alternately, in Sweden where dental health care is
more widespread, individuals with dental caries were infected with
other bacteria such as Actinomyces, Selenomonas, Prevotella, and Capnocytophaga [34]. Possible explanations for the disparity could
include diet as well as hygienic habits. This research suggests that
tooth decay is a common problem in all humans, though the bacteria
responsible may be contextually unique.
In addition to the oral diseases, the oral microbiota has been
linked to neurodegenerative diseases. Diseases such as periodontitis
and dental caries previously discussed can stimulate an inflammatory
response in the body, eventually leading to low-grade systemic
inflammation [35]. This inflammation can easily travel to the blood
vessels, which have been previously shown to have a significant role in
the pathogenesis of neurodegenerative disease. As discussed earlier,
mounting evidence suggested that the blood brain barrier (BBB) is
inadequate to protect the brain from circulating infectious bacteria
from the oral cavity. Moreover, upon entry to the brain, oronasal and
periodontal bacteria do not elicit the normal inflammatory responses
such as meningitis and encephalitis to a noticeable degree to the host
body, and therefore the accumulation of neuronal insult, as well as
the natural onset of immunosenescence leads to the prevalence of
age-related neurodegenerative diseases, such as Alzheimer’s and
Parkinson’s diseases, associated with oral and periodontal diseaserelated
bacteria.
Normal oral bacteria metabolize components of the food that
we eat and release compounds that are then absorbed into the
bloodstream. This metabolism-based relationship between the bacteria
and the host requires a significant amount of immune tolerance on
the part of the host to bacterial secretions. The tolerance provided
by the host, however, presents a substantial risk to bacterial entry to
the bloodstream and subsequent translocation to body areas where
oral bacteria would be detrimental to the surrounding environment.
Additionally, mounting evidence has shown that protective barriers,
of particular importance to this review, the Blood-Brain Barrier
(BBB), are inadequate to prevent this spread of bacteria, leading to
significant risk of neuronal insult from oral and periodontal diseases.
Potential Pathways that Influence Spread
Van Velzen, Abraham-Inpijn, & Moorer published a review
in 1984 in which they determine three mechanistic links between
bacterial load increase due to periodontal and oral disease and
systemic diseases elsewhere in the body, otherwise known as focal
infection [36]. Each pathway presents a potential initiating factor
for circulation of oral bacteria throughout the body, leading to its
deposition in distant organs. These three pathways are:
1. Metastatic infection due to transient bacteremia
2. Metastatic inflammation due to immunological injury
3. Metastatic injury due to microbial toxins
It should be noted that there may be other substantial mechanisms
of focal infection, but research has not illuminated these pathways yet.
Metastatic Infection:
Metastatic infection is the most documented and best-understood
pathway of focal infection and describes the spread of oral microbes
throughout the body as a direct result of bacteremia. As discussed
above as the blood circulation pathway of spread, bacteremia is
typically caused by normal dental hygiene practices such as brushing and flossing in patients with periodontitis or other oral diseases.
Likewise, oral surgery, such as root canals, have also been shown to
cause transient bacteremia [37].In a healthy human, a small amount of transient bacteremia
does not result in long-term systemic inflammation and is usually
cleared by the body within 1 hour of spread. However, if the bacteria
find favorable conditions, such as in the brain, heart, or lungs,
large densities of bacteria tend to localize and begin multiplying.
Particularly, a majority of oral microbes have developed the ability
to strongly adhere to the surfaces of other cells and tissues, an ability
necessary for survival in the tumultuous environment of the oral
cavity, and preset a significantly increased risk of localization when
introduced to the bloodstream.
Metastatic inflammation:
There are numerous substances that are able to pass through the
epithelial barrier and enter the bloodstream, and plaque in the oral
cavity is one such substance. The rate of crossover, particularly in the
gingival sulcular lining of the oral cavity, is dependent on the size of
the molecules, but can also be accelerated by chronic inflammation
of the gums. Therefore, inflammation caused by plaque buildup in
the mouth both presents substantial risk of toxic bacteria to spread
throughout the body, and also acts to facilitate the spread through
chronic inflammation of the oral lining.In addition to the inflammatory response to plaque in the oral
cavity, oral microbes also elicit an inflammatory response due to
shared antigens with the host body. As part of the adaptive immune
response to bacterial infection, a host body will release antibodies
specific to highly-conserved antigens of the infected bacteria.
However, the host body often shares a number of the same antigens
that are targeted by secreted antibodies, thereby causing autoimmune
damage, particularly to the tissues surrounding an area of antibody
localization, and allowing bacterial entry to the bloodstream.
Such self-destructive antibodies are referred to as cross-reacting
antibodies and present a substantial risk to bacteremia in response to
complement activation.
In conjunction with autoimmune damage caused by secreted
antibodies, in some cases where blood borne antigens outnumber
circulating antibodies, intravascular antigen-antibody reactions occur
that cause the formation of macromolecular complexes that continue
to circulate throughout the body. As such immune complexes
circulate, they can begin to localize and deposit throughout the body
and cause acute and chronic inflammatory side effects, increasing the
likelihood of bacterial deposition into distant organs from the mouth.
Metastatic injury:
Microorganisms produce toxins, such as LPS, that exert
significant stress on surrounding tissues and cells. These toxins
appear to be the major cause of most neuronal damage that occurs
as a direct cause of an unhealthy microbiome. Most often neuronal
injury begins in the periphery from circulating bacteria in the blood.
The current consensus follows that bacterial toxins target the myelin
sheaths of peripheral neurons. Chronic neuronal stress from bacterial
endotoxins can then move to the trigeminal ganglion neurons before
eventually affecting significant neuronal changes throughout the central nervous system. As proof of concept, Ratner et. al. showed in
1979 that pain experienced by patients with idiopathic trigeminal or
atypical facial neuralgia was closely related to maxillary or mandibular
bone cavities at the sites of previous tooth extractions where they
discovered a diverse flora of aerobic and anaerobic microorganisms
[38]. This funding directly implemented the oral microbiome in
external symptoms of a nervous system related disease.Beyond the Mouth: 4 Hypotheses of Entry Sites to the Brain
Shoemark & Allen (2015) describe four potential pathways that
oral bacteria use to enter the brain [39]:
1. Blood Circulation
2. The Blood-Brain Barrier
3. The Olfactory Hypothesis
4. Circumventricular Organs and Perivascular Spaces
Each of these four pathways have been implemented in
neuroinflammation leading to neuronal damage. It is currently
unclear if one of these pathway is more prevalent than the others, but
it is clear that each of these pathways has been observed in the spread
of harmful bacteria associated with periodontal disease.
Blood circulation:
During oral and periodontal diseases, the infectious burden
begun in the mouth presents a substantial burden to the rest of the
body, due to the easy spread to the rest of the body from the mouth.
Additionally, certain microorganisms release highly toxic LPS
alongside the cytokine release that may enter the bloodstream during
the inflammatory response. Likewise, daily dental hygiene treatments
such as brushing, chewing, flossing, and using toothpicks introduce
small cuts in the gums and oral lining allowing bacteria to enter into
the bloodstream, a condition known as bacteraemia. This crossover
event presents significant risk to patients with periodontal diseases by
providing harmful anaerobic bacteria a pathway into the bloodstream.
In fact, patients with periodontitis exhibit bacteraemia multiple times
a day, and bacteria have been shown to persist in the bloodstream
for upwards of three hours [2]. Moreover, after spreading to vascular
channels, bacteria have the capacity to spread throughout the body to
the heart, brain, and lungs within one minute.The blood-brain barrier:
The endothelial cells lining the blood capillaries are held together
by tight junctions forming a nearly impenetrable barrier to circulating
blood toxins and bacteria. However, chronic inflammation caused by
periodontal disease-related bacteria can weaken the BBB, allowing
easier access to the brain by harmful bacteria. Additionally, the normal
aging process causes tight junctions at the BBB to loosen, allowing
easier crossover into the brain by bacteria that may cause subsequent
neuronal stress and damage. Compounded over the lifetime, this
neuronal stress can lead to age-related neurodegeneration and
cognitive decline [39].Circumventricular organs and perivascular spaces:
The Circumventricular Organs (CVO), such as the pineal gland, are structures that allow hormones from the hypothalamus to exit the
brain without disrupting the BBB, and are essential to efficient and
proper secretion of hormones throughout the body. Additionally,
the CVOs also function in shuttling substances secreted by organs
elsewhere in the body that would normally not cross the BBB to enter
the brain to enact neuronal changes in response to stimuli from the
periphery. On top of the CVOs, Perivascular Spaces (PVSs) filled with
circulating interstitial fluid that surround the vesicles, also present
easier access to the brain for bacteria than an intact BBB [39].The Olfactory hypothesis:
Many nerves connect the oronasal cavity directly to the brain,
specifically the trigeminal and olfactory nerves. Previous research has
shown that the trigeminal nerve is capable of sustaining measurable
levels of bacteria of the phylum Treponema, a class of obligate
anaerobes that have been implemented in the development of multiple
diseases [40]. Additionally, Olfactory Ensheathing cells (OECs), the
typical line of defense against bacterial infection along the olfactory
tract, have been shown to be inadequate to prevent the crossing over
of bacteria, such as Staphylococcus aureus [41]. Moreover, OECs have
been used to administer nanoparticle drugs to the brain, showcasing
its ability to bypass the BBB entirely [42].Proposed Mechanisms of Neurodegeneration
Knowledge of the pathways used by oral pathogens to spread
to distant organs presents only one facet of the bacterial role in
development of neurodegenerative diseases. Spirochetes, an obligate
anaerobic bacteria associated with periodontal disease has been
implemented frequently in the literature examining bacterial effects
on neurodegeneration. In general, spirochetal infection activates
immune signaling pathways such as toll-like receptor signalling
and the complement cascade. As these pathways progress, the
intermediate products of these pathways mediate the inflammatory
response to bacterial infection, and are often used as biomarkers for
central nervous system inflammation. As part of the natural immune
response, activation of both the innate and adaptive immunities results
in the production of free radicals and apoptosis of infected cells [29].
When present in chronically elevated amounts, bacterial presence
can lead to immune cell exhaustion, as well as overproduction of
free-radicals and excessive apoptosis, which will be discussed later
as prominent mechanisms of bacterial-induced neurodegeneration.
Specifically, the periodontal disease bacteria Porphyromonas gingivalis has been shown to elicit the inflammatory response described above,
but additionally possesses the ability to elude immune factors
and sustain chronic inflammation over long periods of time [43].
Therefore, upon entry into the brain by on me of the previously
described pathways and mechanisms, P. gingivalis induces chronic
inflammation leading to neuronal damage. Depending on the extent
of neuronal damage, the presence of P. gingivalis in the brain may
be quickly detectable by outward symptoms or may amass over the
lifetime and become evident only in elderly patients. This variability
explains the observation of P. gingivalis prevalence in both trigeminal
and atypical neuralgias, as well as in age-related neurodegenerative
diseases, such as Alzheimer’s and Parkinson’s disease.
More specifically, bacterial spread to the nervous system induces
neurodegeneration in two ways: production of free radicals in the form of reactive oxygen species and reactive nitrogen species, and
secretion of endotoxins in the form of endogenous neurotransmitters
secreted in excess. As with most systemic damage, neurodegeneration
begins at the cellular level with these two pathways, and subsequent
cell death causes the spread and intensifying cell damage leading to
the measurable changes in neuron density and observable changes
in neurocognition. Each of these pathways will be looked at more in
depth below.
Formation of free radicals leading to neurodegeneration:
It is well documented that bacterial cell wall components,
particularly LPS, are highly resistant to mammalian enzymes and
degradation pathways, and therefore allow bacteria to induce longterm
infection, and a chronic inflammatory response mediated by
cytokine release. Along with chronic systemic inflammation, cell wall
components of infectious bacteria also induce the formation of free
radicals within infected cells [44]. Free radicals, in elevated levels,
are sufficient to cause cell membrane damage as well as damage to
mitochondrial DNA. The effects of membrane and mitochondrial
damage than cause infected cells to die and undergo apoptosis, thus
releasing free radicals trapped inside the infected cell to cause further
damage to surrounding cells.Excitotoxic release of endogenous neurotransmitters leading to neurodegeneration:
In the normal processes of neuronal activity, excitatory
neurotransmitters, such as glutamate and homocysteine, act to
promote the firing of action potentials and the propagation of
information throughout the nervous system. However, due to
the damage to mitochondrial DNA as discussed above by free
radicals, cellular energy as a whole decreases and neurons become
significantly more sensitive to small amounts of excitatory
neurotransmitters. Under these conditions, even basal levels of
excitatory neurotransmitters can lead to overstimulation of neurons
and neuronal exhaustion from excessive firing. This leads to the
the activation of the p53 gene within overstimulated and exhausted
neurons that signals to the neuron to undergo apoptosis. Therefore,
the free radical mechanism discussed above induces a systemic
response by depleting neuronal energy and causing proliferation of
p53 gene proteins. Accumulation of neuronal damage and cell death
overtime due to chronic infection and over-sensitization of neurons
to stimulation evidences the link between spread of microbes from
the oral cavity with progressive neurodegeneration.Potential Diseases Caused by Spread of Harmful Oral Bacteria
The spread of harmful pathogens from patients with periodontal
disease has been associated with multiple kinds of systemic
infections and diseases. For example, The oral microbes A.
actinomycetemcomitans, P gingivalis [45], Porphyromonas gingivalis, Treponema denticola, Cytomegalovirus and Chlamydophyla pneumonia [46] have all been implemented in the development of
atherosclerosis, all of which are anaerobic microbes. A recent study
found that prolonged periodontal treatment and changing of oral
hygiene habits decreased oral anaerobes, reduced inflammatory
biomarkers, and reversed thickening of the carotid artery associated
with atherosclerosis [47]. However, for the purposes of this review, the following section will focus on the neurodegenerative diseases
Alzheimer’s and Parkinson’s disease, and the role oral pathogens
play in their development using the neurodegenerative mechanisms
described above.
Alzheimer’s disease:
Alzheimer’s disease is the most common form of dementia.
Symptoms include loss of ability to form new memories leading
to confusion, and eventually inability to self-care requiring
institutionalization. Typical age of onset in America is 85 to 89 years
old, but early cases have become more common in recent history.Evidence for an inflammatory response within the AD brain:
Specifically, astrocyte-mediated inflammation evidenced by
increased levels of inflammatory cytokines (TNFα IL-1β). In 2009,
researchers showed that blood levels of TNFα and antibodies for
oral bacteria were significantly higher, as much as 6 times higher,
in Alzheimer’s patients compared to controls. This discovery has
lead to analysis of how these abnormal serum levels might be
used as a diagnostic tool, further evidencing the crucial role the
oral microbiome is playing in the spontaneous pathogenesis of
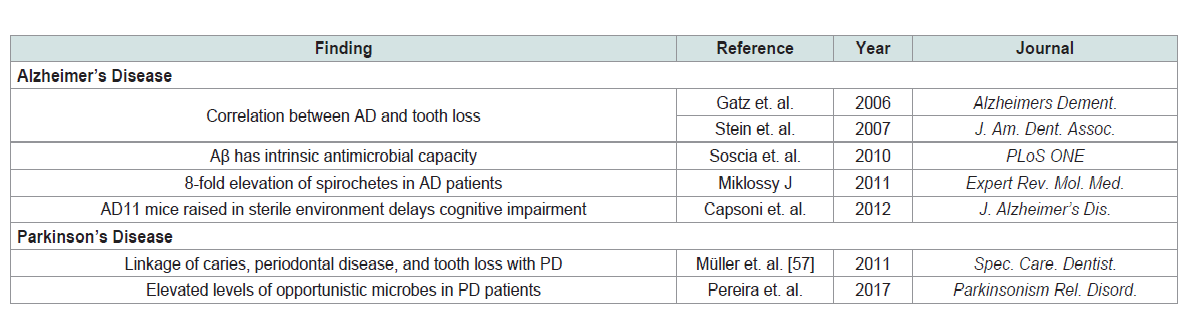
Alzheimer’s disease [48]. Miklossy (2011) found that oral bacteria
were present at a 7-fold increase and with more variety in AD brains
than normal control [49]. Specifically, AD brains contained a large
number of oral spirochetes, obligate anaerobes from the phylum
Treponema (Table 3).The Swedish Twin Registry, begun in the 1950s, found a significant
correlation between dementia and tooth loss before the age of 35 [50].
This was duplicated in a study of North American Nuns [51]. These
correlations assume that early tooth loss is indicative of poor oral
hygiene, and rely on this assumption to evidence the role of the oral
microbiome in these findings.
Additionally, the AD11 mouse model of Alzheimer’s disease has
been shown to produce antibodies which sequester nerve growth
factor throughout their lifetime. This decrease in basal levels of an
essential growth factor slowly removes the support necessary for
proper development of cholinergic cells in the basal forebrain,
leading to the hallmark symptoms of impaired memory, amyloidbeta
and hyperphosphorylated tau lesions, and loss of cholinergic
basal forebrain neurons. Important to this review, when the AD11
mouse model is raised in sterile conditions, the onset of observable
neuropathological changes and cognitive impairment is delayed [52].
It is unclear whether the oral bacteria themselves or secreted
endotoxins, such as LPS, are entering the brain in the pathogenesis
of Alzheimer’s disease. What is clear, however, is that regardless of
in what form the bacteria are exerting their effects on neuronal stress,
the outcome will be microglial activation, specifically of astrocytes,
and the subsequent elevation of TNFα and IL-1β as discussed above.
This claim is also strengthened by the recent discovery that amyloid
beta oligomers, while neurotoxic to the surrounding environment,
also exhibit intrinsic antimicrobial capacity and may reflect an
evolutionary adaptation of the human brain to fight off bacterial
infection [53]. This suggests that elevated bacterial load in the brain
may be the triggering factor leading to elevation of amyloid beta
oligomers and subsequent neurotoxicity from the aggregates left over after the infection has been cleared.
Taken together, this evidence seriously implements the oral
microbiome on the pathogenesis of Alzheimer’s disease. Furthermore,
as humans age, the bacterial load present in the body naturally
increases due to immunosenescence, and the specific microbiome
composition of an individual is becoming increasingly important
for lifelong health. As the human body ages, the innate immune
system predominates the anti-infectious response, and the threat of
increasing bacterial load from unchecked microbiomes intensifies the
importance of maintaining the innate immune barriers, such as the
BBB. This push-and-pull relationship established by early or chronic
periodontal and oral diseases predisposes the aged immune system
to succumb to breach of damaging bacteria, thus accelerating the
accumulation of amyloid beta and amplification of neurocognitive
deficits associated with the Alzheimer’s disease.
Parkinson’s disease:
Parkinson’s disease is an age-related neurodegenerative disease
associated with loss of dopaminergic neurons in the substantia nigra,
as well as the presence of α-synuclein deposits throughout the brain.
Typical outward symptoms of Parkinson’s disease are bradykinesia,
rigidity, and tremors. However, research has not fully illuminated
the exact neuropathological mechanism that leads to the onset of
Parkinson’s disease, but current hypotheses center around the roles
α-synuclein oligomerization, oxidative stress, and mitochondrial
dysfunction. Interestingly, each of these potential risk factors for
Parkinson’s disease have been previously shown to be linked to
transient bacteremia of anaerobic microbes from the oral cavity
and other microbiome localizations. Therefore, it is likely that the
proposed mechanisms of bacterial spread discussed in the previous
sections of this review present potential monitorable early risk factors
for development of Parkinson’s disease [54].Multiple projects in the development of Parkinson’s disease have
involved neuroinflammatory processes, however further research
into the benefits of anti-inflammatory drug treatment have yielded
mixed results [54]. This may be due to a lack of understanding and indepth
analysis of the kinds of anti-inflammatory drugs tested, and so
discrepancies in current research need to be examined more closely.
Most surely, the current body of research relating neuroinflammatory
processes to Parkinson’s disease should not be considered extensive,
and present numerous additional outlooks that must be explored.
To be clear, however, neuroinflammation does not appear to be
the primary cause of cell death in Parkinson’s disease, dissimilar to
Alzheimer’s disease or other neurodegenerative diseases, but instead
presents what can be thought of as a side-effect of the primary pathogenesis of Parkinson’s that acts as a catalyst to accelerate the
deficits characteristic of the disease.
Interestingly, endogenous pathological antigens have been
implemented in the activation of both the innate and adaptive immune
system. As previously described in this review, antigen presence
in the body can elicit damage to the surrounding environment by
either cross-reacting antibodies and intravascular antigen-antibody
complexes. Therefore, it is no stretch to hypothesize that transient
bacteria gaining access to the brain could induce neurodegenerative
effects through one of these two mechanisms [54]. In fact, when
compared to normal human oral microbiome diversity, the oral
microbiomes of Parkinson’s patients displayed elevated levels of
opportunistic microbes, particularly of the phylum Treponema, the
obligate anaerobes implemented in several other neurodegenerative
disorders [55,56]. However, a substantial analysis of bacterial load of
a Parkinson’s brain has not yet been performed, and therefore the
current body of data lacks evidence of microbes associated with the
oral cavity or periodontal disease. However, current research points
to the significant potential that periodontal-disease related bacteria
will be present in higher densities in the Parkinson’s brain.
Looking to the future, further knowledge of the microbiomes
present within the human body, as well as their interactions with
the human genome, will improve both the validity of diagnosis of
neurodegenerative diseases, as well as provide potential risk factors
that can be used for early prediction and attenuation of symptoms
prior to the current clinically diagnosable onset. Furthermore,
accumulation of data on these interactions will accelerate the clinical
research into candidate drugs and therapies to aid in the treatment of
neurodegenerative diseases.
Concluding Remarks
While microbiota tend to have a bad reputation, and there is
substantial research outlining the plethora of harmful infections
that can be caused by rampant spread of this bacteria, it is important
to remember that humans and bacteria have coevolved ever since
the dawn of humanity. The connection we share is complex and is
clearly both good and bad depending on the type, prevalence, and
location of these bacteria. To dictate that the microbiota housed
in our bodies are entirely commensalistic or even parasitic would
neglect this relationship that we have developed. While the roles of
some bacteria remain unknown and the degree to which some may
cause neurodegenerative diseases requires further research, their
importance to maintaining human life is evident and must not be
forgotten.
References
22. Tesmer LA, Lundy SK, Sarkar S, Fox DA (2008) Th17 cells in human disease.
Immunol Rev 223: 81-113.