Journal of Oral Biology
Download PDF
Review Article
Decision Tree to Minimize Intraoperative Complications during Maxillary Sinus Augmentation Procedures
Nezar Boreak*, Panpicha Maketone, Julien Mourlaas, Wendy CW Wang and Paul YC Yu
- Department of Periodontology and Implant Dentistry, New York University, USA
*Address for Correspondence: Nezar Boreak, Department of Periodontology and Implant Dentistry, New York University, Clinic 5W, 345 E 24th St,New York, NY 10010, USA, Tel: +1-646-715-5230; E-mail: Nmb428@nyu.edu
Citation: Boreak N, Maketone P, Mourlaas J, Wang WCW, Yu PYC. Decision Tree to Minimize Intra-operative Complications during Maxillary Sinus Augmentation Procedures. J Oral Biol. 2018; 5(1): 8.
Copyright: © 2018 Boreak N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 5, Issue: 1
Submission: 15 January, 2018 | Accepted: 30 April, 2018 | Published: 07 May, 2018
Keywords
Sinus augmentation; Intra-operative complication; Decision tree; Dental implant
Abstract
Rehabilitation of patients with endosseous implants in the posterior maxilla poses a challenge as a result of sinus pneumatization and ridge atrophy. Sinus augmentation has been established to be a reliable procedure to facilitate implant therapy. However, despite the predictability of the techniques and biomaterials employed in sinus graft procedures, intra-operative complications still occur leading to increased surgical time, abortion of the surgery, post-operative infections and loss of implant. Based on the clinical findings and literature review, a hierarchical decision tree to minimize intra-operative complication is proposed. Several host-related factors, which can influence the outcome of the procedure, are identified. The factors are health status of the sinus, size and location of the endosseous anastomosis, lateral wall thickness, membrane thickness, remaining residual bone height, and presence of sinus floor cortication.
Introduction
Rehabilitation of patients with endosseous implants in the posterior maxilla poses a challenge as a result of sinus pneumatization and ridge atrophy. The introduction and development of the sinus augmentation procedure has greatly facilitated implant therapy in this region [1-3]. In 1996, the Sinus Consensus Conference concluded that sinus graft should be considered as highly predictable and effective therapeutic modality [4].
Extensive research has since been conducted on the types of bone graft materials, the types of implants, and timing for implant placement for sinus graft. Implant survival rates have been reported to range from 81% to 95% depending on the combination of graft materials and implant surfaces [5]. Implants with rough surfaces have been shown to have similar survival rates to implants placed in nongrafted sites irrespective of the type of sinus grafting materials [7]. Simultaneous implant and graft placement has been demonstrated to be predictable provided mechanical anchorage of the dental implant is achieved [7,8].
The Sinus Consensus Conference was revisited in 2016 and reaffirmed the validity of the sinus graft [9]. It concluded that non-inductive materials with slow resorption might be superior in forming and maintaining bone than inductive materials [10-14]. The consensus also questioned the need for biologic enhancement with growth factors and morphogenic proteins [10].
Despite the predictability of the techniques and biomaterials employed in sinus graft procedures, intra-operative complications like Schneiderian membrane perforation, excessive bleeding and reduced implant stability are common [15-17]. Additional postoperative consequences include increased surgical time, abortion of the surgery, post-operative infections and loss of implant into the sinus. Strong correlation exists between these complications and individual anatomical variations. Membrane thickness, shape of the sinuses, and presence of antral septa are possible factors affecting Schneiderian membrane perforation [18-20]. Lack of precision in identifying the presence, diameter and location of the endosseous branch of the posterior superior alveolar artery are commonly associated with excessive bleeding [21].
Poor bone quality and inadequate residual crestal bone height can result in loss of primary stability does not allow for simultaneous implant placement [22].
The host’s inherent factors play an important role in the success of sinus graft. The purpose of this review is to establish an evidencebased hierarchical checklist of critical host factors essential to minimize intra-operative complication for predictable maxillary sinus floor augmentation.
Materials and Methods
A search of the literature was performed focusing on complications related to sinus augmentation procedures, anatomical variations from components of the maxillary sinus and recommendations on simultaneous implant placement. Clinical data in this study was obtained from the anonymous Implant Database (ID) at the Ashman Department of Periodontology and Implant Dentistry at the New York University College of Dentistry. This data was extracted as de-identified information from the routine treatment of patients. Yes, most of the studies involving human subjects found on the database search are included. The ID was certified by the Health Insurance Portability and Accountability Act (HIPAA) and approved by the University Committee on the Activities Involving Human Subjects (UCAIHS). A computer search of electronic database from MEDLINE and PUBMED at the Waldman Library at the NYUCD was performed. Keywords such as “maxillary sinus”, “sinus lift”, “sinus augmentation”, “complications”, “dental implant”, “simultaneous placement” were used, alone and in combination, to search the databases. Non-English language publications were excluded. The search was limited to studies involving human subjects. Restrictions were not placed regarding the type of study design.
Result
The results of this review are based on literatures review and presented in Table 1. These are assimilated to form a hierarchical decision tree to minimize intra-operative complication for lateral wall sinus augmentation technique presented in Table 1. The hierarchy is based on the anticipated severity of the medical and dental complications.
Clinical Case
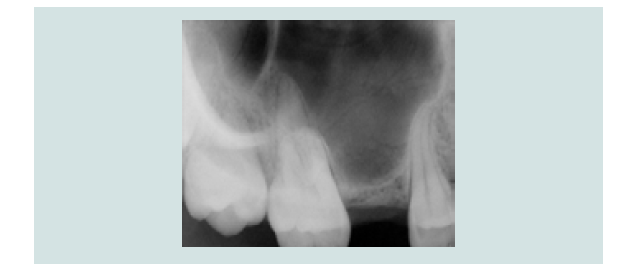
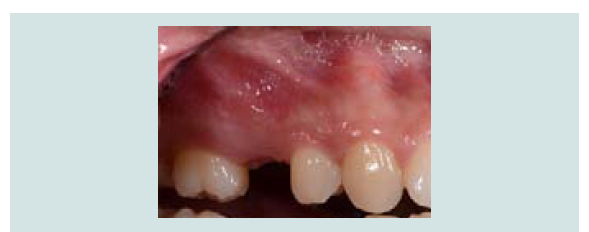
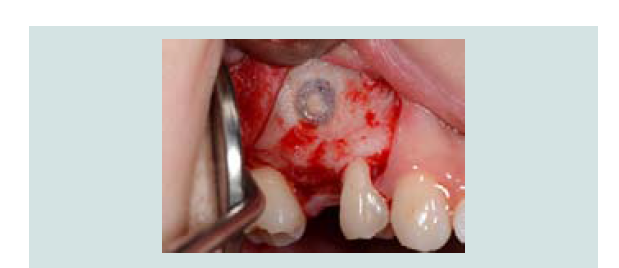
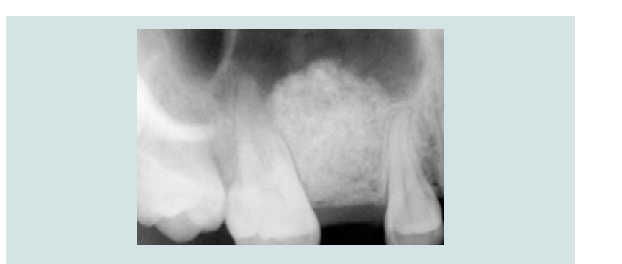
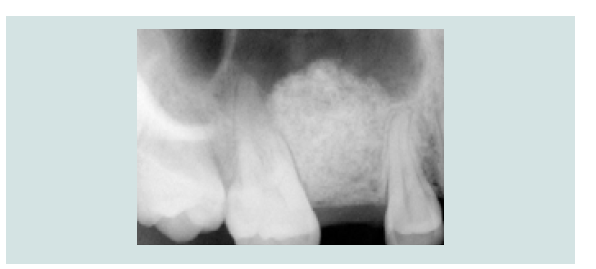
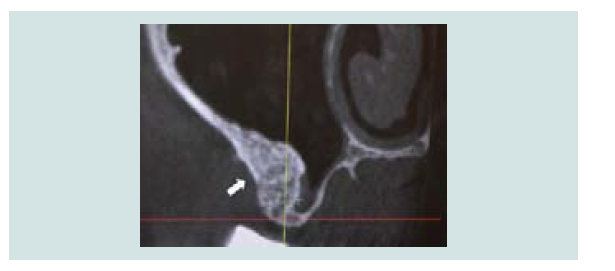
A 26 year-old healthy female patient with absence of sinus pathology presented with a missing maxillary right second premolar. The tooth was extract 3 years ago due to failed root canal treatment. Pre-operative periapical radiograph and Cone Beam Computed Tomography (CBCT) assessment showed an atrophic edentulous ridge with residual bone height between 2-3 mm (Figure 1A). Intra-oral assessment revealed adequate 3-dimensional space for restoration (Figure 1B).
Figure 1A: Pre-operative periapical radiograph with less than 3mm of residual crestal bone height at the surgical site.
The patient received 2 g amoxicillin one hour before surgery. Following administration of local infiltration anesthesia (2% lidocaine with epinephrine 1:100,000), a crestal incision and two vertical releasing incisions on mesial aspect of maxillary right first premolar and distal aspect of maxillary first molar were placed, and a full thickness mucoperiosteal flap exposing the lateral wall of the sinus was reflected.
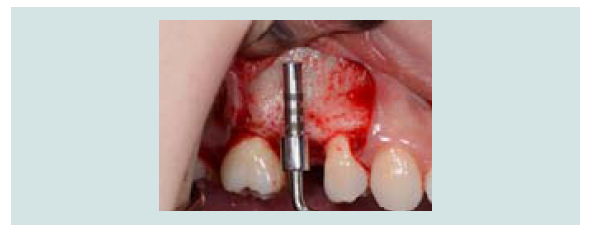
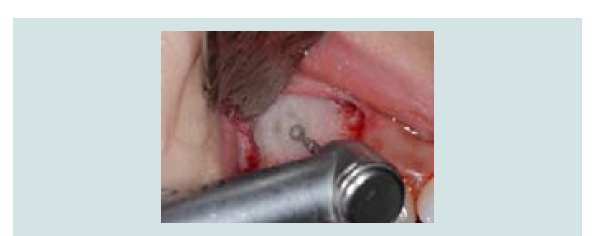
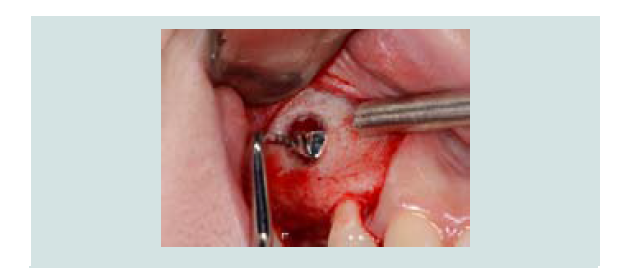
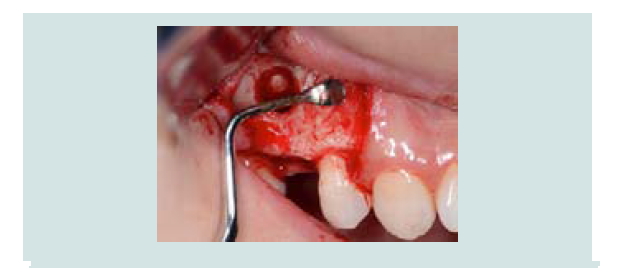
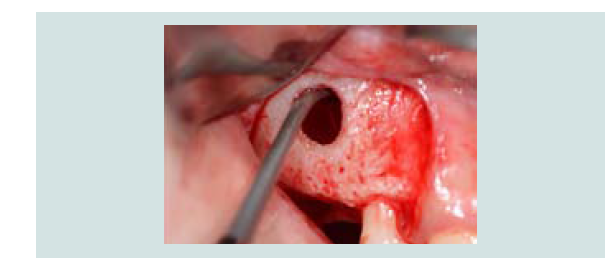
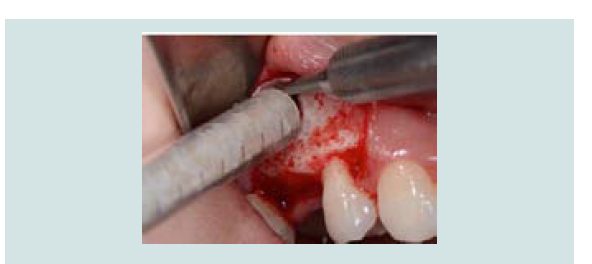
A measuring device (SCC4, EBI, South Korea) was used to simulate the implant position and the anticipated level of membrane elevation (Figure 1C). An oval-shaped window osteotomy in the lateral sinus wall was created with a round high speed diamond bur under copious irrigation (Figures 1D and 1E). The sinus membrane was then elevated through osteotomy window with a sinus membrane elevator which, placed between the membrane and the edge of the window to gently tease out the membrane (Figure 1F). The instrument was placed in contact with the underlying sinus bony wall throughout elevation to avoid perforation. Once the membrane is elevated from the underlying bone, the anterior border of the membrane was located and elevated (Figure 1G), followed by the elevation of medial wall for maximum graft nutrient (Figure 1H).
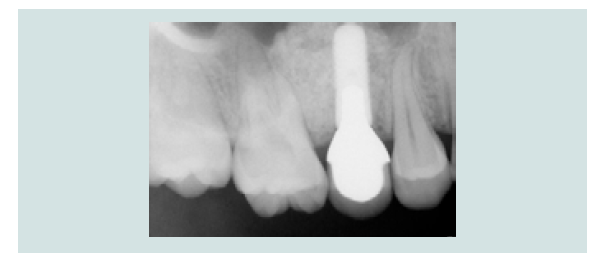
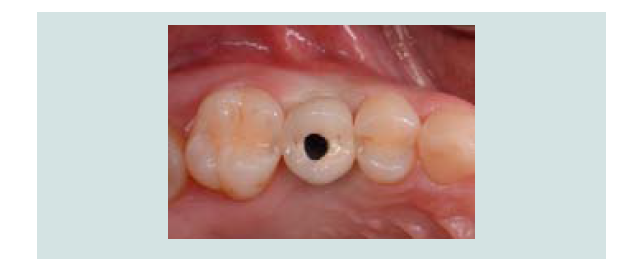
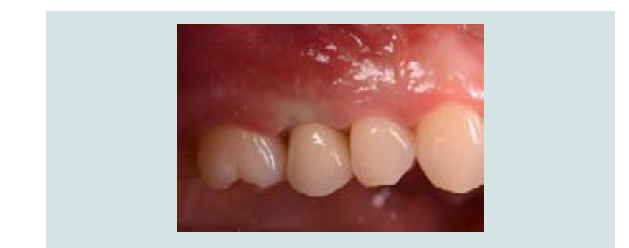
Following the completed elevation (Figure 1I), anorganic bone graft was compacted (Bio-Oss, Geistlich Pharma, Switzerland) into the sinus cavity with a bone syringe and gently packed with a hand instrument (Figures 1J and 1K). Sinus graft placement was confirmed with a periapical radiograph (Figure 1L). A 4.1x12 mm implant (RC, Straumann, Switzerland) was placed 4 months following the sinus graft (Figure 1M). A screw-retained restoration was delivered 2 months after implant placement (Figures 1N-1P).
Discussion
The ultimate goal of the sinus augmentation technique is to increase the available bone height for implant placement. This is accomplished by the sequential steps of flap elevation, window access to the sinus cavity, elevation of the Schneiderian membrane to create a confined space, graft placement and flap closure. However, sinus grafts and implant placement being elective procedures, all care must be taken to avoid alteration of patient’s physiological sinus function and well-being. Intra-operative complications are predominately due to surgical difficulties encountered during the course of the procedure. The authors would like to made general statement on complication after reviewing several articles, such as “degrees of complication range from life threatening such as uncontrollable bleeding, cavernous sinus infection to minor perforation.
Health condition of the sinus
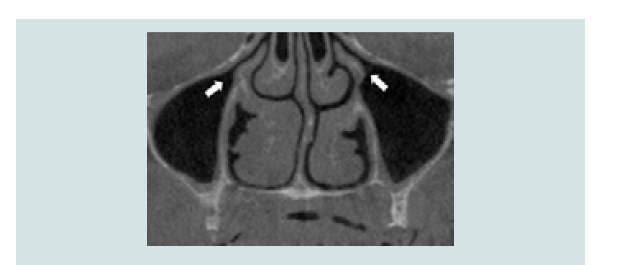
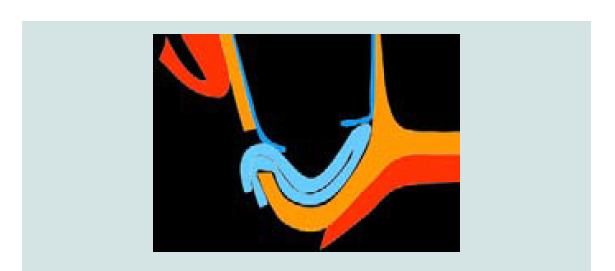
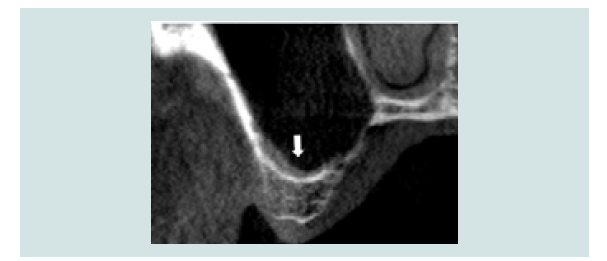
Absence of sinus pathology is the prerequisite for sinus graft. Any pre-existing condition that may disturb the patency of drainage or containment of the sterile graft has to be recognized and addressed. Drainage of the maxillary sinus is through the ostium, which is positioned in the superior medial aspect of the sinus and opens into the nasal cavity between the middle and lower nasal conchae (Figure 2). Elevation of the Schneiderian membrane on the medial wall is recommended to increase blood supply to the graft [23]. When mucosal thickening is present, elevation of this membrane may lead to the obstruction of the drainage resulting in an altered anatomy of the maxillary sinus and subsequent changes in function [24].
Figure 2: CT-scan image with arrows indicating the position and patency of the ostium in the superior medial aspect of the sinus.
Mucosal thickening is common with documented findings ranging from 23.3% to 56.6% (Figure 3) [25,26]. Multiple causes have been linked to mucosal thickening and they are generally associated with some form of irritation, such as inflammation or allergic phenomena [27]. In its localized form, it is most likely associated to odontogenic infections, particularly apical infections [27,28].
When a focal or extended thickening is less than 2 mm, sinus graft can be performed without the need for prior exploration [24]. It is recommended to refer to an Otorhinolaryngologist (ENT) prior to sinus graft procedure to restore it to its physiologic state in cases of moderate to severe mucosal thickening associated with a history of sinusitis or if half of the sinus is filled [24].
Other less frequent findings of sinus pathology also call for ENT consultation prior to sinus graft. Mucoceles are due to obstruction of the sinus ostium and drainage pattern leading to accumulation of mucus within the sinus cavity. Continual accumulation and expansion can lead to erosion of sinus wall [29]. Differential diagnosis for expansion and bone destruction includes malignant conditions and must be ruled out prior to sinus graft. On the other hand, an opaque maxillary sinus without bone erosion invites the diagnosis of sinusitis, retention cysts, and antrochoanal polyps [29]. Mucous retention cysts are not uncommon [27]. Large mucous retention cysts may result in compromised drainage and should be addressed prior to sinus graft. Small mucus retention cyst can be readily detected and can be drained at the time of surgery with a large gauge needle.
Endosseous anastomosis at osteotomy site
A thorough understanding and identification of the vascular supply to the sinus cavity is essential prior to sinus graft. Although life-threatening hemorrhage is rare, excessive bleeding can hinder visibility, increase the risk of intra-operative complications and distress both the patients and clinicians [30].
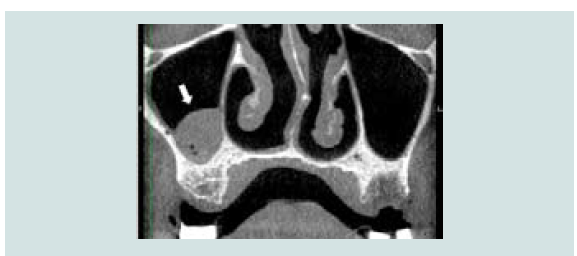
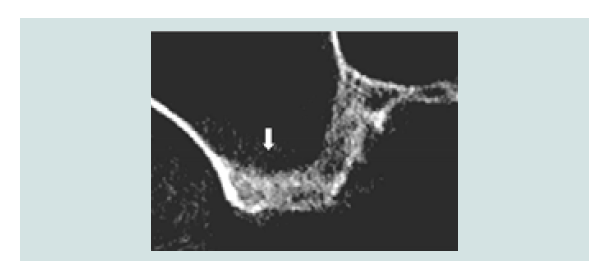
Vascularization of the antero-lateral wall of the sinus is characterized by the anastomoses of the posterior superior alveolar artery and the infra-orbital artery inside and outside the bony lateral antral wall (Figure 4). Solar and colleagues demonstrated endosseous anastomosis is always present, whereas an extraosseous anastomosis is identified in about 44% of the cases [31]. However, detection rate of endosseous anastomosis dropped to an average of 61% in computer tomography studies and may be dependent on the experiences of the clinicians [32]. As endosseous anastomosis can run superficially, intra-osseously or intra-sinusly and may not be constantly detectable throughout its course, all sagittal cuts of the CT must be evaluated (Figure 5) [33].
Figure 5: Sagittal cross-section of CT-scan showing presence of endosseous anastomosis (> 2 mm) of the posterior superior artery in the lateral wall of the sinus.
The diameter of the endosseous anastomosis is less than 1 mm in 55% -71% cases making its identification difficult in CT-scan examination [21,33-35]. These anastomoses have the potential for bleeding complications in 20% of cases due to their location [21]. The risk of hemorrhage is as high as 57% when the diameter is between 1-2 mm which is found in 30-40% of endosseous anastomosis [32,33,36]. Diameter of more than 2 mm is rare with reported finding between 1-4% [33,34]. Piezosurgery has been advocated as a safer approach compared to diamond high-speed bur as it enables dissection of the artery [37]. However, care must be taken as the dissected artery could still be wounded during the membrane elevation. Furthermore, our clinical experiences found that controlling of excessive bleeding in patients with a long-term use of anticoagulant can be challenging even with cessation of medication prior to surgery. A thorough medication history ought to be taken including usage of supplement. Some off the counter supplements when taken in high dosages have been shown to increase bleeding tendency [38]. Therefore, a transcrestal or palatal approach should be considered in patient presenting with a large diameter endosseous sinus artery in combination with a long-term use of anticoagulant.
Bleeding can also occur from trauma to the extraosseous branch in the soft tissue during vertical releasing incisions for flap elevation or via periosteal releasing incisions for flap enhancement. Periosteal releasing incision is usually not necessary for sinus graft procedure unless a barrier membrane is used to cover the window osteotomy.
Recent meta-analysis showed that the use of a barrier membrane does not influence the amount of vital bone formation [39]. Clinicians must weigh the treatment goal against longer treatment time and possible complication. Often time, clinicians are not aware of the presence of hemorrhage until the flap is closed, particularly where the flap mucosa is thick and the anastomosis is deeply embedded. Immediate post-operative swelling of the surgical site and continuous blood seepage from the suture line requires immediate attention.
Lateral wall thickness
The lateral wall thickness, composition and shape of sinus cavity should be evaluated in regard to choice of surgical approach. Mean lateral wall thickness in relation to position on the maxillary arch has been recently documented. The mean thickness of the lateral wall of the maxillary sinus was 1.21±1.07 mm at the second molar, 1.98±1.87 mm at the first molar, 2.02±1.53 mm at the second premolar and 2.16±1.25 mm at the first premolar [40]. Precise CBCT measurement of wall thickness coupled with the utilizing of a diamond bur of a known diameter for window osteotomy is a useful technique. It permits intra-operative appreciation of the location of the Schneiderian membrane from the bur, reducing incidences of membrane perforation.
Performing a complete osteotomy where the lateral wall is thick (e.g. >3 mm) and the zygomatic buttress is prominent is time consuming, as access to the upper border of the window is restricted. Once inside the sinus cavity, good access and good vision are necessary to facilitate membrane elevation. Ability to maneuver the instrument for membrane elevation can be hindered by a thick lateral wall. Often, the window needs to be enlarged to ensure the instrument is at the right angulation when in contact with the bony wall of the sinus. In addition, the thicker the wall, the higher chance of a larger portion of the cancellous bone in its composition. Cancellous bone is more vascularized compare to cortical bone. The increased bleeding may obstruct visibility and prolong the surgical procedure [36].
When accesses to the antral cavity from the lateral wall are contraindicated, entry to the sinus cavity transcrestally or palatally are the viable alternatives. An indication for palatal approach is illustrated in Figure 14 where the existing sinus graft is inadequate and more graft is needed via a re-entry procedure (Figure 6) [3,41].
Schneiderian membrane thickness
Schneiderian membrane perforation is the most common intra-operative complication associated with sinus augmentation procedures [42]. Frequency rates ranging from 11% up to 56% have been reported [41,43]. Membrane perforation has been related to a higher post-operative infection incidence as it threatens the coverage and containment of the bone graft [44,45].
The correlation between membrane thickness and perforation rate during sinus augmentation procedures have been recently reviewed. The perforation rate was lowest when the thickness was 1.5-2 mm for transcrestal sinus lift and lowest when the membrane thickness was 1-1.5 mm for lateral approach [18,45]. Therefore, a pathology free, resilient membrane with thickness between 1-2 mm supports a predictable sinus augmentation procedure, and caution should be exercised when membrane is thin (<1 mm) or thick (>2 mm) to minimize the incidence of perforation [46].
Anatomical variations such as presence of underwood septa or a V shape sinus cavity are other risk factors for membrane perforation as they obscure visibility and limit access to the antral space [19,45]. The occurrence of the septa has been documented in 20% to 35% of cases [20].
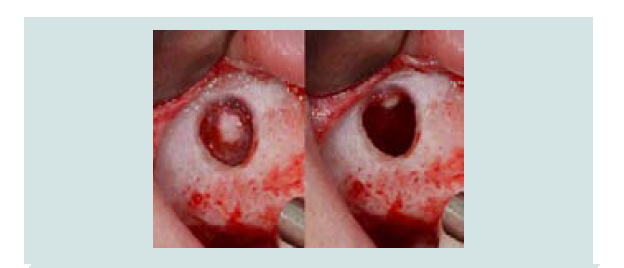
Multiple management procedures have been described from aborting the surgery to suturing the wounded membrane [47-49]. A predictable two-stage approach technique to manage large perforations has been recently described in a case series by Dagba AS et al. [50]. Once a large perforation occurred, further elevation of the membrane is ceased and a collagen sponge is folded and placed at the perforation site which acts as a space maintainer and provides a scaffold for cells recruitment to the wounded area (Figure 7) [51]. Sinus augmentation procedure is postponed 3-6 weeks after repair of the perforation. This time frame allows the membrane to heal, facilitating for easier re-entry (Figure 8) [52].
Residual crestal bone height and timing of implant placement
The residual crestal bone height (RBH) is an indicator to decide on the surgical procedure for sinus graft. RBH of 5 mm or less is considered for lateral window technique [53]. The recommended RBH threshold is based on the limited bone gain from the transcrestal osteotome technique in comparison to the lateral approach. Both lateral window or transcrestal approaches are applicable when the RBH is 6 mm or more. It has been suggested that the transcrestal approach is less invasive however it is a blind technique in comparison to the lateral approach where direct visualization and manipulation of the membrane is possible [3].
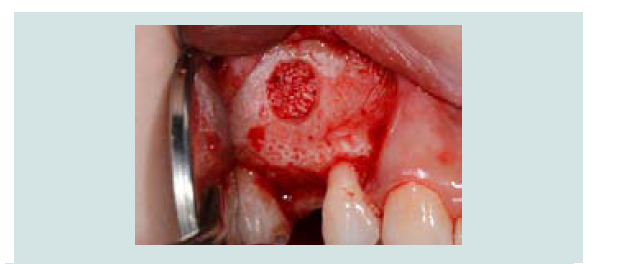
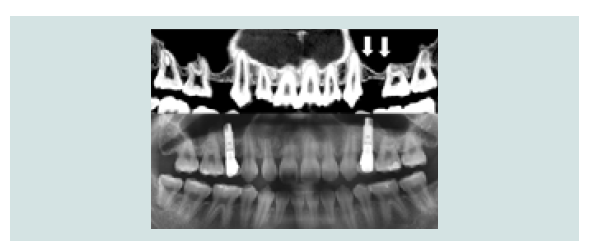
A minimum of 5 mm RBH is traditionally recommended for simultaneous surgical procedure to ensure adequate implant stabilization and parallelism; when the RBH is less than 4 mm, delayed implant placement is traditionally advocated [54]. Simultaneous implant placement with inadequate RBH may increase the risk of implant migration into the sinus cavity following initial bone remodeling. Peleg M et al. reported no statistically significant difference in the implant survival rate placed simultaneously in various RBH provided primary stability could be achieved [55]. Primary stability can be increased by under drilling; engaging internal anatomy wherever possible, or tilting implant placement to engage more native bone if prosthetic design permits [56]. Furthermore, RBH is not uniform anteriorly posteriorly often, primary stability can be achieved by engaging the uneven RBH. Figure 9 demonstrated simultaneous implant placements at premolar sites where the uneven sinus floor allows for improved implant anchorage.
The shape of the sinus cavity should be evaluated. A higher membrane perforation rate is demonstrated in V-shaped sinuses where the angle between the lateral wall and the medial wall is less than 30 degrees [19]. The acute angle makes it more difficult to angulate the hand instrument leading to higher chances of perforation; whereas less perforation is noted in U shape cavity where maneuvering the hand instrument is easier. However, it should be noted that more bone to implant contacts might be expected in a V-shaped cavity than U-shaped cavity when all other parameters are equal [19]. The closer distance to the medial wall also allows for better blood supply and faster maturation of the graft (Figure 10).
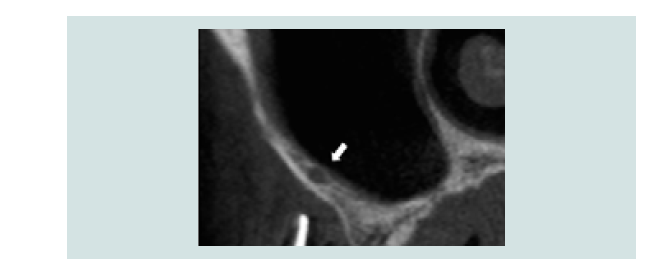
Sinus floor cortication
Bi-cortical anchorage has been advocated to provide higher primary stability as multiple layers of dense bone are engaged by the implant. A recent radiographic classification of the sinus floor cortication has been introduced by Choucroun G et al. [56]. Options for sinus augmentation and timing of implant placement have been proposed based on the four-cortication groups described. Favorable recommendation has been made by the authors for lateral augmentation and simultaneous implant placement where cortication is present which is found in 72% of the CBCT scans studied (Figure 11). Simultaneous implant placement should be avoided where sinus floor cortication was absent unless there is adequate RBH to achieve primary stability (Figure 12).
Conclusion
The study reviewed the factors affecting sinus augmentation and their clinical implications. The predictability of the sinus augmentation and implant placement procedure relies more on the inherent host factors and not only on the biomaterials. The checklist provides guidelines for treatment planning in augmenting the atrophic maxilla to minimize intra-operative complication.
Acknowledgements
Special thanks to Dr. Giovanni Molinas Rojas, DDS and Dr. Asmita R Bhekare, BDS for their continuation of clinical cases and revision of the manuscript.
References
- Tatum H Jr (1986) Maxillary and sinus implant reconstructions. Dent Clin North Am 30: 207-229.
- Boyne PJ (2006) History of maxillary sinus grafting. In Jensen OT (Ed.) The sinus bone graft (2ndedn). Quintessence Publishing Co, Hanover Park, Illinois, USA, pp 735.
- Summers RB (1994) A new concept in maxillary implant surgery: the osteotome technique. Compendium 15: 152-158.
- Jensen OT, Shulman LB, Block MS, Iacono VJ (1998) Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants 13 Suppl: 11-45.
- Aghaloo TL, Moy PK (2007) Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants 22 Suppl: 49-70.
- Pjetursson BE, Tan WC, Zwahlen M, Lang NP (2008) A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J Clin Periodontol 35 (8 Suppl): 216-240.
- Felice P, Pistilli R, Piattelli M, Soardi E, Barausse C, et.al (2014) 1-stage versus 2-stage lateral sinus lift procedures: 1-year post-loading results of a multicenter randomised controlled trial. Eur J Oral Implantol 7: 65-75.
- Johansson LA, Isaksson S, Lindh C, Becktor JP, Sennerby L (2010) Maxillary sinus floor augmentation and simultaneous implant placement using locally harvested autogenous bone chips and bone debris: a prospective clinical study. J Oral Maxillofac Surg 68: 837-844.
- Jensen O, Block MS, Iacono V (2016) 1996 Sinus Consensus Conference revisited in 2016. Int J Oral Maxillofac Implants 31: 505-508.
- Del Fabbro M, Testori T, Francetti L, Weinstein R (2004) Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int J Periodontics Restorative Dent 24: 565-577.
- Froum SJ, Wallace SS, Cho SC, Elian N, Tarnow DP (2008) Histomorphometric comparison of a biphasic bone ceramic to anorganic bovine bone for sinus augmentation: 6-to 8-month postsurgical assessment of vital bone formation. A pilot study. Int J Periodontics Restorative Dent 28: 273-281.
- Froum SJ, Tarnow DP, Wallace SS, Rohrer MD, Cho SC (1998) Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic, and histomorphometric analysis--Part 2 of an ongoing prospective study. Int J Periodontics Restorative Dent 18: 528-543.
- Froum SJ, Wallace SS, Elian N, Cho SC, Tarnow DP (2006) Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent 26: 543-551.
- Testori T, Wallace SS, Trisi P, Capelli M, Zuffetti F, et al. (2013) Effect of xenograft (ABBM) particle size on vital bone formation following maxillary sinus augmentation: a multicenter, randomized, controlled, clinical histomorphometric trial. Int J Periodontics Restorative Dent 33: 467-475.
- Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, et al. (1997) A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent 17: 11-25.
- Zijderveld SA, van den Bergh JP, Schulten EA, ten Bruggenkate CM (2008) Anatomical and surgical findings and complications in 100 consecutive maxillary sinus floor elevation procedures. J Oral Maxillofac Surg 66: 1426-1438.
- Chan HL, Wang HL (2011) Sinus pathology and anatomy in relation to complications in lateral window sinus augmentation. Implant Dent 20: 406-412.
- Wen SC, Lin YH, Yang YC, Wang HL (2015) The influence of sinus membrane thickness upon membrane perforation during transcrestal sinus lift procedure. Clin Oral Implants Res 26: 1158-1164.
- Cho SC, Wallace SS, Froum SJ, Tarnow DP (2001) Influence of anatomy on Schneiderian membrane perforations during sinus elevation surgery: three-dimensional analysis. Pract Proced Aesthet Dent 13: 160-163.
- Wen SC, Chan HL, Wang HL (2013) Classification and management of antral septa for maxillary sinus augmentation. Int J Periodontics Restorative Dent 33: 509-517.
- Elian N, Wallace S, Cho SC, Jalbout ZN, Froum S (2005) Distribution of the maxillary artery as it relates to sinus floor augmentation. Int J Oral Maxillofac Implants 20: 784-787.
- Marquezan M, Osório A, Sant'Anna E, Souza MM, Maia L (2012) Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin Oral Implants Res 23: 767-774.
- Wallace SS, Tarnow DP, Froum SJ, Cho SC, Zadeh HH, et al. (2012) Maxillary sinus elevation by lateral window approach: evolution of technology and technique. J Evid Based Dent Pract 12 (3 Suppl): 161-171.
- Friedland B, Metson R (2014) A guide to recognizing maxillary sinus pathology and for deciding on further preoperative assessment prior to maxillary sinus augmentation. Int J Periodontics Restorative Dent 34: 807-815.
- Maestre-Ferrín L, Galán-Gil S, Carrillo-García C, Peñarrocha-Diago M (2011) Radiographic findings in the maxillary sinus: comparison of panoramic radiography with computed tomography. Int J Oral Maxillofac Implants 26: 341-346.
- Manji A, Faucher J, Resnik RR, Suzuki JB (2013) Prevalence of maxillary sinus pathology in patients considered for sinus augmentation procedures for dental implants. Implant Dent 22: 428-435.
- Rege IC, Sousa TO, Leles CR, Mendonça EF (2012) Occurrence of maxillary sinus abnormalities detected by cone beam CT in asymptomatic patients. BMC Oral Health 12: 30.
- Vallo J, Suominen-Taipale L, Huumonen S, Soikkonen K, Norblad A (2010) Prevalence of mucosal abnormalities of the maxillary sinus and their relationship to dental disease in panoramic radiography: results from the health 2000 health examination survey. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109: e80-e87.
- Sreedharan S, Kamath MP, Hegde MC, Bhojwani K, Alva A, et al. (2011) Giant mucocoele of the maxillary antrum: a case report. Indian J Otolaryngol Head Neck Surg 63: 87-88.
- Hong YH, Mun SK (2011) A case of massive maxillary sinus bleeding after dental implant. J Oral Maxillofac Surg 40: 758-760.
- Solar P, Geyerhofer U, Traxler H, Windisch A, Ulm C, et.al (1999) Blood supply to the maxillary sinus relevant to sinus floor elevation procedures. Clin Oral Implants Res 10: 34-44.
- Valente NA (2016) Anatomical considerations on the alveolar antral artery as related to the sinus augmentation surgical procedure. Clin Implant Dent Relat Res 18: 1042-1050.
- Rosano G, Taschieri S, Gaudy JF, Weinstein T, Del Fabbro M (2011) Maxillary sinus vascular anatomy and its relation to sinus lift surgery. Clin Oral Implants Res 22: 711-715.
- Hayek E, Nasseh I, Hadchiti W, Bouchard P, Moarbes M, et al. (2015) Location of Posterosuperior alveolar artery and correlation with maxillary sinus anatomy. Int J Periodontics Restorative Dent 35: e60-e65.
- Mardinger O, Abba M, Hirshberg A, Schwartz-Arad D (2007) Prevalence, diameter and course of the maxillary intraosseous vascular canal with relation to sinus augmentation procedure: a radiographic study. Int J Oral Maxillofac Surg 36: 735-738.
- Ella B, Sédarat C, Noble Rda C, Normand E, Lauverjat Y, et al. (2008) Vascular connections of the lateral wall of the sinus: surgical effect in sinus augmentation. Int J Oral Maxillofac Implants 23: 1047-1052.
- Wallace SS, Mazor Z, Froum SJ, Cho SC, Tarnow DP (2007) Schneiderian membrane perforation rate during sinus elevation using piezosurgery: clinical results of 100 consecutive cases. Int J Periodontics Restorative Dent 27: 413-419.
- Grisa A, Florio S, Bellia E, Cho SC, Froum SJ (2016) The role of dietary supplements in postsurgical bleeding: An update for the practitioner. Compend Contin Educ Dent 37: 690-695.
- Suárez-López Del Amo F, Ortega-Oller I, Catena A, Monje A, Khoshkam V, et al. (2015) Effect of barrier membranes on the outcomes of maxillary sinus floor augmentation: a meta-analysis of histomorphometric outcomes. Int J Oral Maxillofac Implants 30: 607-618.
- Danesh-Sani SA, Movahed A, ElChaar ES, Chong Chan K, Amintavakoli N (2016) Radiographic evaluation of maxillary sinus lateral wall and posterior superior alveolar artery anatomy: a cone-beam computed tomographic study. Clin Implant Dent Relat Res 19: 151-160.
- Sarmiento HL, Othman B, Norton MR, Fiorellini JP (2016) A Palatal approach for a sinus augmentation procedure. Int J Periodontics Restorative Dent 36: 111-115.
- Zijderveld SA, van den Bergh JP, Schulten EA, ten Bruggenkate CM (2008) Anatomical and surgical findings and complications in 100 consecutive maxillary sinus floor elevation procedures. J Oral Maxillofac Surg 66: 1426-1438.
- Kasabah S, Krug J, Simůnek A, Lecaro MC (2003) Can we predict maxillary sinus mucosa perforation? Acta Medica (Hradec Kralove) 46: 19-23.
- Nolan PJ, Freeman K, Kraut RA (2014) Correlation between Schneiderian membrane perforation and sinus lift graft outcome: a retrospective evaluation of 359 augmented sinus. J Oral Maxillofac Surg 72: 47-52.
- Schwarz L, Schiebel V, Hof M, Ulm C, Watzek G, et al. (2015) Risk factors of membrane perforation and postoperative complications in sinus floor elevation surgery: review of 407 augmentation procedures. J Oral Maxillofac Surg 73: 1275-1282.
- Lin YH, Yang YC, Wen SC, Wang HL (2016) The influence of sinus membrane thickness upon membrane perforation during lateral window sinus augmentation. Clin Oral Implants Res 27: 612-617.
- Becker ST, Terheyden H, Steinriede A, Behrens E, Springer I, et al. (2008) Prospective observation of 41 perforations of the Schneiderian membrane during sinus floor elevation. Clin Oral Implants Res 19: 1285-1289.
- Proussaefs P, Lozada J (2003) The “Loma Linda Pouch”: a technique for repairing the perforated sinus membrane. Int J Periodontics Restorative Dent 23: 593-597.
- Testori T, Wallace SS, Del Fabbro M, Taschieri S, Trisi P, et al. (2008) Repair of large sinus membrane perforations using stabilized collagen barrier membranes: surgical techniques with histologic and radiographic evidence of success. Int J Periodontics Restorative Dent 28: 9-17.
- Dagba AS, Mourlaas J, Ochoa Durand D, Suzuki T, Cho SC (2015) A novel approach to treat large Schneiderian membrane perforation-a case series. Int J Dent Oral Health 6: 1.
- Luitaud C, Laflamme C, Semlali A, Saidi S, Grenier G, et al. (2007) Development of an engineering autologous palatal mucosa-like tissue for potential clinical applications. J Biomed Mater Res B Appl Biomater 83: 554-561.
- Burkhardt R, Lang NP (2014) Fundamental principles in periodontal plastic surgery and mucosal augmentation--a narrative review. J Clin Periodontol 41 Suppl 15: S98-S107.
- Khouly I, Rivero DG, Perez A, Khouly S, Cho SC, et al. (2014) Treatment options for the atrophic posterior maxilla. Actualidad médica 99: 152-156.
- Smiler DG, Johnson PW, Lozada JL, Misch C, Rosenlicht JL, et al. (1992) Sinus lift grafts and endosseous implants. Treatment of the atrophic posterior maxilla. Dent Clin North Am 36: 151-186.
- Peleg M, Garg AK, Mazor Z (2006) Predictability of simultaneous implant placement in the severely atrophic posterior maxilla: a 9-year longitudinal experience study of 2132 implants placed into 731 human sinus grafts. Int J Oral Maxillofac Implants 21: 94-102.
- Choucroun G, Mourlaas J, Kamar Affendi NH, Froum SJ, Cho SC (2017) Sinus floor cortication: classification and prevalence. Clin Implant Dent Relat Res 19: 69-73.