Journal of Oncobiomarkers
Download PDF
Gelatinase zymography
Research Article
*Address for Correspondence: Aleksandra Niedzwiecki, Oncology Division, Dr. Rath Research Institute 1260 Memorex Drive, Santa Clara, CA-95035, USA, Tel: 408-567-5050; Fax: 408-567-5030; E-mail: author@drrath.com
Citation: Roomi MW, Bhanap B, Niedzwiecki A, Rath M. Suppression of Matrix Metalloproteinases -2 and -9 in Various Human Cancer Cell Lines by a Nutrient Mixture. J Oncobiomarkers. 2015;2(1): 17.
Copyright © 2013 Niedzwiecki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oncobiomarkers | ISSN: 2325-2340 | Volume: 2, Issue: 1
Submission: 13 January 2015 | Accepted: 21 February 2015 | Published: 26 February 2015
Reviewed & Approved by: Dr. Peiwen Fei, Associate Professor of Cancer biology, University of Hawaii Cancer Center, USA
Abbreviations: MMP: Matrix Metalloproteinases; NM: Nutrient Mixture; ECM: Extracellular Matrix; PMA: Phorbol 12-Myristate 13-Acetate; ER: Estrogen Receptor; EGCG: Epigallocatechin Gallate
Cancer cell lines and reagents
Suppression of Matrix Metalloproteinases -2 and -9 in Various Human Cancer Cell Lines by a Nutrient Mixture
M Waheed Roomi, Bilwa Bhanap, Aleksandra Niedzwiecki* and Matthias Rath
- Oncology Division, Dr. Rath Research Institute, 1260 Memorex Drive, Santa Clara, CA 95050, USA
*Address for Correspondence: Aleksandra Niedzwiecki, Oncology Division, Dr. Rath Research Institute 1260 Memorex Drive, Santa Clara, CA-95035, USA, Tel: 408-567-5050; Fax: 408-567-5030; E-mail: author@drrath.com
Citation: Roomi MW, Bhanap B, Niedzwiecki A, Rath M. Suppression of Matrix Metalloproteinases -2 and -9 in Various Human Cancer Cell Lines by a Nutrient Mixture. J Oncobiomarkers. 2015;2(1): 17.
Copyright © 2013 Niedzwiecki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oncobiomarkers | ISSN: 2325-2340 | Volume: 2, Issue: 1
Submission: 13 January 2015 | Accepted: 21 February 2015 | Published: 26 February 2015
Reviewed & Approved by: Dr. Peiwen Fei, Associate Professor of Cancer biology, University of Hawaii Cancer Center, USA
Abstract
The matrix metalloproteinases (MMPs) are a family of zinc containing endopeptidases that degrade various components of the extra cellular matrix. Among the many types of MMPs that have been identified, MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are thought to play a key role in cancer metastasis. These MMPs are able to modify tumor microenvironment by degrading type IV collagen found in the cellular basement membrane. MMP-2 and -9 are essential in facilitating cancer cell invasion, tumor progression, and metastasis, thereby shortening patient survival in all cancer types. A significant association has been reported between tumor aggression and increased levels of MMP-2 and MMP-9. Therefore, MMPs are used as diagnostic and prognostic biomarkers in many clinical trials and experimental studies. MMP inhibitors (MMPIs) seem to be the logical targets for the therapeutic intervention in cancer. The rationale for developing MMPIs for halting cancer has been around for more than three decades. However, the clinical trials of synthetic MMPIs have not produced favorable results.In this review, we summarize the results of our in vitro studies evaluating effects of a natural and non-toxic nutrient mixture on inhibition of the cancer biomarkers; MMP-2 and MMP-9 in the treatment and control of metastasis of 42 different cancer cell lines from 13 representative classes of malignancies.
Abbreviations: MMP: Matrix Metalloproteinases; NM: Nutrient Mixture; ECM: Extracellular Matrix; PMA: Phorbol 12-Myristate 13-Acetate; ER: Estrogen Receptor; EGCG: Epigallocatechin Gallate
Introduction
Cancer is the second leading cause of death in the world, striking people of all ages [-3]. Yearly cancer deaths are projected to increase to over 11 million deaths worldwide by 2030. On an individual level, the overall risk of developing cancer during one’s lifetime is 50% for men and 33% for women [1,2]. The most frequent cancers in men are lung, prostate, and colorectal cancer, in that order, while in women, the cancers with highest incidence are breast, cervical, and colorectal cancer. The highest mortality is associated with lung, stomach, and liver cancer [1-4].The treatment regimen used for a specific cancer depends primarily on the type of cancer and the stage of cancer. For most cancers, surgery, radiotherapy, and chemotherapy are preferred treatment modalities. However, all three options are associated with several short and long-term detrimental side effects for the patient [4,5]. Chemotherapy and radiotherapy indiscriminately attack healthy as well as cancer cells, causing extensive damage throughout the body. Moreover, analysis of the clinical trials from 1990 to 2004 on 22 types of cancers showed that chemotherapeutic intervention increases the five-year survival rate by a mere 2.1% [1-4]. Currently, researchers are exploring targeted compounds for cancer treatments to reduce wide spread adverse effects.
Cancer cells secrete zinc-dependent endopeptidases, the matrix metalloproteinases (MMPs), which remodel tumor’s surrounding microenvironment by cleaving collagen fibers and other extracellular matrix components, thereby facilitating metastasis [6-18]. More than 90% of the cancer deaths are associated with metastatic cancer [4-10].
Therefore, finding a safer and effective alternative to inhibit the action of MMP enzymes would potentially avoid side effects and damage to the healthy cells, while treating cancer patients.
Therefore, finding a safer and effective alternative to inhibit the action of MMP enzymes would potentially avoid side effects and damage to the healthy cells, while treating cancer patients.
Although approximately 25 different MMPs act on a broad spectrum of substrates, including collagen type I, II, III, and IV, and stromyelin, of these MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are particularly attractive targets of MMP-2 and MMP-9 inhibitors (MMPI) research because of their central role in tumor metastasis [5-15]. MMP-2 and MMP-9 play prominent roles in the degradation of type IV collagen found in the basement membrane and extracellular matrix [16-21]. MMP-2 and MMP-9 have the ability to modify the tumor microenvironment, a complex extracellular matrix network that mediates interactions between tumor cells, stromal cells, and immune cells. Alterations in the tumor microenvironment can cause changes in local metabolism, tumor growth, tumor progression, treatment resistance, and, eventually, metastatic potential. Indeed, a significant association has been reported between both experimental and clinical tumor aggression and increased levels of MMP-2 and MMP-9. Thus, the challenge is to develop an effective MMP inhibitor that utilizes minimally toxic and readily absorbed naturally derived substances, which slow or inhibit MMP-2 and MMP-9 action.
Numerous studies have found that certain naturally derived substances individually exhibit potent inhibitory effects on the progression of a wide variety of cancers [22-43]. The NM is a combination of several nutrients formulated to target the key physiological pathways in cancer progression and metastasis. For example, the ECM integrity is dependent upon adequate collagen formation and its stability. In this aspect ascorbic acid and the amino acids, lysine and proline are necessary for the formation and optimum structure of collagen fibers. Manganese and copper are also essential cofactors in collagen formation process. Collagen stability can be controlled by lysine [29] and by N-acetyl cysteine (NAC) through its inhibitory effect on MMP-9 activity [30] and invasiveness of tumor cells [31]. Ascorbic acid, among other anti cancer actions, is also proven to inhibit cancer cell division and growth through production of hydrogen peroxide [32-37]. Green tea extract is known to be a promising agent in controlling angiogenesis, metastasis, and other aspects of cancer progression [38,39]. In addition, selenium has been shown to interfere with MMP expression and tumor invasion and to induce selective apoptosis of cancer cells [40,41]. Since, arginine is a precursor of nitric oxide (NO); any deficiency of arginine can limit the production of NO, which predominantly acts as an inducer of apoptosis [42]. Furthermore, we have reported that the effects of a specific combination of these nutrients were superior to their individual effects or their random combination on anti- proliferative action on cancer cells [43].
The research from our group has also shown that combining these naturally derived substances achieves a synergistic effect against MMP-2 and MMP-9 secretion [44,49]. This synergistic nutrient mixture (NM) used in this review is a combination of several nutrients formulated to target the key physiological pathways in cancer progression and metastasis.
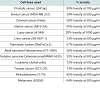
We studied the effect of NM on the secretion of MMP-2 and MMP-9 by 42 cancer cell lines using gelatinase zymography ([table 1,figure 1]). The cancer cell lines studied were grouped by organ systems such as female breast and gynecological cancers, testicular, prostate and male breast cancers, gastrointestinal cancers, lung cancer, mesothelioma, head and neck cancers, pediatric sarcomas, adult sarcomas, leukemias, and others, to determine the therapeutic potential of NM in preventing metastases of these cancers. Figure 1 demonstrates the secretion of MMP-2 and MMP-9 in melanoma cell lines A 2058, with and without PMA (100 ng/ml).
Methods and Materials
42 different cancer cell lines were selected based on different patterns of MMP-2 and MMP-9 secretion, and included various carcinomas, adenosarcomas, soft tissue sarcomas, and leukemias [table 1]. The cancer cell lines and their recommended media were purchased from ATCC (Rockville, MD, USA). All other reagents, including fetal bovine serum (FBS), penicillin, streptomycin, phorbol 12-myristate 13-acetate (PMA), were of high grade and obtained from Sigma (St. Louis, MO). All other reagents used were of high purity and were obtained from Sigma, unless otherwise indicated.
Composition of the nutrient mixture (NM)
The nutrient mixture (NM) includes the following: Vitamin C (as ascorbic acid and as Mg, Ca and palmitate ascorbate) 710 mg; L-lysine 1000 mg; L-Proline 750 mg; L-Arginine 500 mg; N-Acetyl Cysteine 200 mg; Standardized Green Tea Extract (80% polyphenol) 1000 mg; Selenium 30 μg; Copper 2 mg; and Manganese 1 mg.
Cell culture
Cancer cell lines were grown in recommended media, supplemented with 10% FBS, penicillin (100 units/mL), and streptomycin (100 μg/mL) in 24-well tissue culture plates (Coster, Cambridge, MA, USA). The cells were plated at a density of 1 X 105cells/mL and grown to confluence in a humidified atmosphere at 5% CO2 at 37 °C. At near confluence, serum-supplemented media were removed and the cell monolayer was washed once with PBS and with the recommended serum-free medium. The cells were then treated with the nutrient mixture (NM), dissolved in media, and tested at 0, 10, 50, 100, 500, and 1000 μg/mL in triplicate at each dose. Parallel sets of cultures were treated with PMA (100 ng/ml) for induction of MMP-9. The plates were then returned to the incubator. The conditioned media were collected separately, pooled, and centrifuged at 4 °C for 10 min at 3000 rpm to remove cells and cell debris. After 24 hours, the media were removed and the supernatant was collected and used for gelatinase zymography, which is a highly sensitive assay of gelatinolytic enzymatic activity able to detect both pro and active forms of MMP-2 and MMP-9.
Gelatinase zymography was performed in 10% NOVEX Pre-Cast SDS Polyacrylamide Gel (Invitrogen Corporation) in the presence of 0.1% gelatin under non-reducing conditions. Culture media (20 μL) were mixed with sample buffer and loaded for SDA-PAGE with trisglycine SDS buffer as suggested by the manufacturer (Novex). Samples were not boiled before electrophoresis. Following electrophoresis, the gels were washed twice in 2.5% Triton X-100 for 30 min at room temperature to remove SDS. The gels were then incubated at 37°C overnight in substrate buffer containing 50 mM Tris-HCl and 10 mM CaCl2 at pH 8.0 and stained with 0.5% Coomassie Brilliant Blue R250 in 50% methanol and 10% glacial acetic acid for 30 min and destained. Upon renaturation of the enzyme, the gelatinases digest the gelatin in the gel and give clear bands against an intensely stained background. Protein standards were run concurrently and approximate molecular weights were determined by plotting the relative mobilities of known proteins. Gelatinase zymograms were scanned using a CanoScan 9950F Canon scanner at 300 dpi. The intensity of the bands was evaluated using the pixel-based densitometer program Un-Scan-It, Version 5.1, 32-bit, by Silk Scientific Corporation (P.O. Box 533, Orem, UT 84059, USA), at a resolution of 1 Scanner Unit (1/100 of an inch for an image that was scanned at 100 dpi). The pixel densitometer calculates the optical density of each pixel (values 0 to 255) using the darkly stained background of the gel as a pixel value of zero. A logarithmic optical density scale was used since the optical density of films and gels is logarithmically proportional to the concentration. The pixel densitometer sums the optical density of each pixel to give a band’s density. In all graphs, band densities were reported as percentages of the sums of all pixels in a given lane of a gel.
The effect of the NM on MMP secretion was assessed by treating cells to increasing concentrations of NM and expressed as relative band densities (as % of control value). The cumulative effect of NM was determined by summing all the band densities within a treatment group and comparing these densities between treatment groups. The extent of total MMP band secretion in a treatment group was reported as ‘total band pixel.
Our earlier studies have shown that combining these naturally derived substances achieve a substantially more potent synergistic effect against constitutive and PMA-stimulated MMP-2 and MMP- 9 secretion than any action of any of the individual nutrients [44-49]. Previous research from our institute has shown that nutrients such as ascorbic acid, lysine, and epigallocatechin gallate when tested individually have anti-proliferative and apoptotic effects on different types of leukemia cells [62-64]. While there could be other possible mechanisms, according to our previous studies when L-lysine and EGCG were separately used in in HTLV-1 positive and –negative leukemia cell, it was proven that these nutrients exert their effects through Nuclear Factor-kappaB (NF-kB) pathway [64]. Similarly, EGCG also has apoptotic action on leukemia cells and it inhibits the MMP-9 expression on both transcriptional and translational levels[64].
Given the fundamental role of MMP-2 and MMP-9 in the progression of cancer, the dose-dependent suppression of MMP- 2 and MMP-9 secretion demonstrates the enormous potential of this combination of natural compounds in cancer treatment. Development of synthetic MMP inhibitors has been underway for more than thirty years. Yet, none of the numerous synthetic MMP inhibitors has been proven successful beyond the phase of clinical testing due to their poor absorption, bioavailability and undesirable side effects during the administration and with long term usage [5]. Since secretion of MMPs is crucial to the invasive potential of cancer cells, the research in MMP inhibitors continues.
Results
Female cancers (breast and other gynecological cancers)Breast cancer:
It is the most common cancer diagnosed in women and is the second leading cause of deaths, after lung cancer, in women in the world. Breast cancer, when diagnosed early, can be treated using surgery, radiation, chemotherapy, or hormonal therapy. There is no complete cure and the 5-year survival of all patients with breast cancer is 88% when given appropriate treatment [1-4,50,52].
We investigated the effect of NM on estrogen receptor (ER) positive and ER negative breast cancer cell lines; MCF-7 and MDAMB- 231, respectively. Neither of the cell lines showed any secretion of MMP-2 even after stimulation by PMA. However, both cell lines secreted MMP-9 upon PMA stimulation. NM treatment resulted in a dose-dependent suppression of MMP-9 secretion in both cell lines. Exposure of ER negative MDA-MB-231 at 50 μg/mL NM concentration resulted in a complete suppression of MMP-9 secretion and in the in MCF-7 the secretion of MMP-9 was inhibited at 500 μg/ mL NM ([table 2].
Cervical cancer:
It is the second most common cancer of the female reproductive tract and is currently the third leading cause of cancer death in women. More than 85% of deaths due to cervical cancer occur in developing countries and the reduced mortality in the developed countries is mostly attributed to regular pap smear testing. The incidence of cervical cancer is highest among women with a history of sexually transmitted diseases [1-4].
We investigated the effect of NM on cervical cancer cell line, HeLa, which secreted MMP-2 and MMP-9, with and without PMA stimulation. The NM decreased levels of both enzymes in a dosedependent manner both in the presence and in absence of PMA. A complete inhibition of MMP-2 secretion occurred at 500 μg/mL NM and MMP-9 at 10 μg/mL NM ([table 1,figure 2a,figure 2b]. Cervical cancer cell line DoTc2-4510 secreted only MMP-9, with responded to PMA stimulation. NM inhibited MMP-9 secretion in a dosedependent manner, with its complete inhibition at NM concentration of 500 μg/ml.
Ovarian cancer:
This is the deadliest of the gynecological cancers, and is the fifth leading cause of cancer death among US women. The lethal nature of ovarian cancer is due to its ability to metastasize rapidly. In most cases, the cancer is already advanced at the time of diagnosis. Currently, 50% of the women diagnosed with ovarian cancer die from that cancer within five years [1-4,50].
We investigated the NM effect in vitro on the human ovarian cancer cell line SK-OV-3, which secreted MMP-2 after PMA stimulation. NM inhibited MMP-2 secretion in a dose-dependent manner with its complete inhibition at 50 μg/mL NM concentration [table 2].
Unlike cervical cancer, uterine cancer is twice as common in developed countries. Uterine sarcomas are a rare type of cancer that accounts for 5% of all uterine malignancies. The most common type is a carcinosarcoma arising from the endometrium and from the muscle cells. The second most common accounting for 30% of all uterine sarcomas is a leiomyosarcoma, which has a peak incidence at age 50. Sarcomas arising from the stroma of the endometrium account for 15% of all uterine sarcomas.
We investigated the in vitro effect of NM on the leiomyosarcoma cell line SK-UT-1, the uterine sarcoma cell line MES-SA, and on multidrug resistant uterine sarcoma cell line MES-SA/DX5. SK-UT-1 did not secrete either MMP-2 or MMP-9 constitutively. MMP-9 secretion in this cell line was induced after PMA treatment, both, active and inactive forms. This cell line exposed to NM showed dose-dependent inhibition of MMP-9 secretion with complete inhibition at 1000 μg/ ml. MES-SA and MES-SA/DX5 secreted only MMP-2 constitutively MMP-9 secretion required stimulation by PMA. Secretion of MMP- 2 and MMP-9 by MES-SA and MES-SA/DX5 cells, both before and after treatment with PMA, was inhibited by NM in a dose-dependent manner with complete suppression occurring at a NM concentration of 10-100 μg/ml [table 2].
Male cancers (prostate, testicular, male breast cancer)Prostate cancer:
Prostate cancer is the most common cancer in men and second most deadly cancer in the US [1-4], primarily affecting males 55 years and older. Prostate cancer is more common in African-American males than white males and occurs more frequently in developed countries. Developed countries account for 75%; the highest rates are found in Europe, North America, and Australia. Current diagnostic methods, e.g. PSA testing and digital rectal exam have helped in early detection of prostate cancer. Standard treatment of consists of surgery (prostatectomy), hormonal therapy, and radiotherapy.
We investigated the effect of NM in vitro on two androgeninsensitive human prostate cancer cell lines, PC-3 and DU-145, and one androgen-sensitive cell line, LNCaP. Results demonstrate that Du-145 cell line does not secrete MMP-2 or MMP-9. PMA stimulated the secretion of MMP-9 in DU-145. MMP-9 secretion is inhibited by NM in a dose-dependent manner, with a complete inhibition at 500 μg/mL NM [table 3]. The androgen insensitive cell line PC-3 secrets both MMP-2 and MMP-9 with and without PMA stimulation. In both cases, NM can inhibit MMP-2 and MMP-9 secretion in a dosedependent manner with complete inhibition occurring at 100-500 μg/ mL NM [table 3,figure 3a,figure 3b]. The androgen-sensitive cell line LNCaP secreted neither MMP-2 nor MMP-9 in the absence or presence of PMA.
Testicular cancer:
It is one of the most frequently occurring cancers in young men. The incidence in whites is greater than in African-American males. Peak frequency is in early adulthood, between the ages of 20-35, and uncommon after the age of 40. Risk factors for testicular cancer include undescended testes, Klinefelter syndrome, and HIV-positive patients. Chemotherapy has been shown to eradicate testicular cancer in a number of cases and life expectancy is long [1-4].
We investigated the effect of NM on the secretion of MMPs in human testicular cancer cell line NTER-2. Results show that this cell line constitutively secretes only MMP-2, and MMP-9 secretion can be achieved under PMA stimulation. Whether with or without PMA stimulation, both MMP-2 and MMP-9 secretion decreased by the NM in a dose-dependent manner, with a complete inhibition at 100- 500 μg/mL NM concentration [table 3].
Male breast cancer:
Breast cancer is less common in men and constitutes less than 1% of all breast cancers. The incidence of male breast cancer is increasing and infiltrating ductal carcinoma is the most common type of male breast cancer. However due to less breast tissue in males, the breast cancer is already spread to distant organs at the time of diagnosis, reducing the chances of complete recovery.
We investigated the effect of NM on MMP secretion in male breast cancer cell line colo-824. This cell line showed only one band corresponding to MMP-9, which was enhanced by PMA stimulation.
MMP-9 secretion was inhibited by NM in a dose dependent fashion showing a faint band at 500 μg/ml and virtually 100% inhibition at 1000 μg/ml (data not shown.
Lung cancer and mesotheliomaLung cancer:
Lung cancer is the most common form of cancer diagnosed in the US, and has one of the lowest survival rates due to high metastatic potential and poor results with standard treatments [1-4]. Lung cancer rates are increasing throughout the world, including Asia, South America, and Africa. The risk factors include cigarette smoking, occupational exposure to asbestos, radon, chromium, nickel, and diesel exhaust.
We investigated the effect of the NM on the human lung cancer cell line A-549. Constitutive MMP-2 and MMP-9 secretion, as well as PMA-induced MMP-9 secretion, was inhibited with NM treatment in a dose-dependent manner with complete MMP suppression occurring at a NM concentration of 500 μg/ml (Table 4; Figures 4A and 4B).
Malignant mesothelioma:
Malignant mesothelioma is a highly aggressive tumor that arises from mesothelial lining of pleural cavities. Due to its extensive metastatic potential at the time of diagnosis, the median survival for patients with malignant mesothelioma is estimated to be 4 to 18 months. Epidemiological evidence suggests that, due to heavy use of asbestos until 1980, the number of men in Western Europe dying due to malignant mesothelioma each yearwill increase from 5,000 in 1998 to 9,000 in 2018. Single and multiple treatment modalities, such as surgery, chemotherapy, and radiation have failed to substantially alter this statistic [1-4].
We investigated the effect of NM on human malignant mesothelioma cell line MSTO-211H. NM significantly inhibited MMP-2 and MMP-9 secretion with and without PMA treatment in a dose-dependent manner. Given the lack of viable treatment options for this highly aggressive cancer, it is particularly significant that complete suppression of MMP secretion in MSTO-211H was achieved at a NM concentration of 500 μg/ml [table 4].
Gastrointestinal cancers (colon, hepatocellular, pancreatic)Colon cancer:
Colon cancer, the second leading cause of cancer death in the United States, causes 55,000 deaths per year. More than 90% of colon cancer occurs in individuals over 50 years old. Risk factors include obesity, smoking, and consumption of alcohol, red meat, fatty diets, diets low in fiber, and diets low in calcium. Surgery is effective in only about 50% of the cases and the five-year survival rates are between 50-60%.
We investigated the effect of NM on colon cancer cell line HCT-116. HCT-116 did not secrete MMP-2 either constitutively or with PMA stimulation; MMP-9 was secreted constitutively. The NM inhibited MMP-9 secretion in a dose-dependent manner with complete suppression occurring at 100 μg/ml NM concentration (table 5).
Liver cancer:
Primary liver cancer, which originates predominantly from the parenchymal liver cells or hepatocytes, includes hepatocellular carcinoma, angiosarcoma, cholangiocarcinoma, and hepatoblastoma. Hepatocellular carcinoma (HCC) is the most predominant, accounting for 90% of liver cancers. Hepatitis B and C viruses increase the risk of primary liver cancer. Other risk factors include excessive alcohol consumption and exposure to chemicals such as aflatoxin B1, androgenic steroids, and oral contraceptives. Other types of liver cancer are less common.
We investigated the effect of NM on two human HCC cell lines, Hep-G2 and SK-Hep-1. NM inhibited secretion of both MMP-2 and MMP-9 in the presence and absence of PMA, in a dose-dependent manner. For both cancer cell lines, a complete inhibition of MMP-2 and MMP-9 secretion occurred at a NM concentration of 500-1000 μg/m (table 5,figure 5a,figure 5b).
Pancreatic cancer:
Cancer of the pancreas pancreatic cancer is the thirteenth common cancer in the world. Although uncommon, pancreatic cancer is the fourth leading cause of cancer-related deaths in both men and women and is almost always fatal. Pancreatic cancer causes approximately 28,000 deaths in the US and 50,000 deaths in Europe each year.
We investigated the effect of NM in vitro on human pancreatic cancer cell line MIA-PaCa-2. MIA-PaCa-2 does not secrete MMP-2 either constitutively or with PMA stimulation. However, secretion of MMP-9 was inhibited by the NM in a dose-dependent manner with its complete suppression at 1000 μg/mL (table 5).
Head and neck cancer (laryngeal, tongue, thyroid)
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy in the US and has a yearly incidence of 40,000 to 50,000 cases [1-4]. Ninety percent of these cancers involve squamous cell histology. The most common sites are the oral cavity, pharynx, larynx, and hypopharynx. HNSCC are known for their aggressive growth and propensity to invade and metastasize. Smoking tobacco, chewing tobacco, and alcohol are major risk factors for developing HNSCC. Other risk factors include sun exposure, occupational exposure to nickel, radium, chromium, mustard gas, leather, wood dust, radiation, EBV infections, and disorders of DNA repair such as Fanconi anemia. HNSCC is one of the major causes ofmortality and morbidity in Fanconi anemia (FA) patients.
We investigated the antineoplastic effects of NM on human FA HNSCC cell line cell line OHSU-974. Zymography revealed MMP- 2 and PMA-induced MMP-9 secretion. NM suppressed secretion of both MMPs in a dose-dependent manner with virtual inhibition at 500 μg/ml.
The NM was also effective in affecting MMP secretion in laryngeal squamous cell carcinoma (FaDu), tongue carcinoma (SCC- 25), and thyroid carcinoma (SW-579). Both MMP-2 and MMP-9 were inhibited in a dose-dependent manner before and after PMA stimulation in FaDu (table 6) and SCC-25 (table 6,figure 6a,figure 6b). The same dose-dependent inhibition was observed in SW-579 only for MMP-2 since this cell line did not secrete MMP-9 even after PMA stimulation. Complete suppression of MMP secretion in FaDu required a NM concentration of 500-1000 μg/ml and 1000 μg/m in both SCC-25 and SW-579 (table 6).
Sarcomas
Adult sarcomas:(Liposarcoma, fibrosarcoma, chondrosarcoma, synovial sarcoma) Liposarcoma, a malignancy of fat cells, is the most common soft tissue sarcoma with an annual incidence of 2.5 cases per million. Fibrosarcoma, an aggressive and highly metastatic cancer of the connective tissue, primarily develops in the metaphyses of long tubular bones and affects both children and adults. Chondrosarcoma, a malignant tumor of cartilage cells mainly affecting adults between 30-60 years and accounting for more than 40% of the adult primary bone tumor cases. The poor prognosis associated with fibrosarcoma and chondrosarcoma can be attributed to both, the aggressive metastatic spread characteristic of these cancers and lack of efficacy of current treatments [1-4].
We investigated the effect of NM in vitro on chondrosarcoma cell line SW-1353, fibrosarcoma HT-1080, liposarcoma SW-872, and synovial sarcoma SW-982. The secretion of MMP-2 and MMP- 9, both constitutive and PMA-stimulated, was inhibited in a dosedependent manner by NM with complete suppression requiring a NM concentration of 500-1000 μg/ml (table 7). The complete suppression of MMP secretion in the particularly aggressive fibrosarcoma line HT-1080 is a particularly encouraging result (table 7,figure 7a,figure 7b).
Pediatric sarcomas (Osteosarcoma, rhabdomyosarcoma):
Malignancies of the soft tissues (6%) and bone (5%) account for more than 10% of diagnosed cancers in children, adolescents, and young adults. Benign musculoskeletal neoplasms are more common than malignant soft tissue tumors [1-4]. Rhabdomyosarcoma, the most common soft tissue sarcoma, is found predominantly in males. It is mesenchymal in origin, and affects primarily infants and children up to five years of age. On the other hand, osteosarcoma accounts for about 60% of malignant bone tumors between the ages of 10-20 years of age. The remaining bone malignancies in children and adolescents are Ewing sarcoma, a soft tissue cancer that most often occurs around the leg or arm joint and has a 50% rate of metastasis.
We investigated the effect of NM on osteosarcoma cell lines MNNG-HOS, SK-ES-1, and U-2OS, and rhabdomyosarcoma cell line RD. All the four cell lines secreted MMP-2 and MMP-9 constitutively and under PMA stimulation. The NM inhibited MMP-2 secretion in a dose-dependent manner with its complete suppression at 500 μg/ ml (Table 8). U-2OS and RD cells secreted MMP-9 constitutively.MMP-9 secretion in these cancer cell lines was much more sensitive to suppression by the NM requiring as little as 10 μg/ml of NM for complete inhibition. MNNG-HOS and SK-ES-1 cell lines secreted MMP-9 only after PMA stimulation, which was inhibited by NM in a dose-dependent manner with a complete inhibition at 500 μg/ml NM (table 8).
Renal and urinary cancers (kidney, bladder)
Renal cancer: The American Cancer Society estimates approximately 65,150 new cases of kidney cancer in 2013. Upon diagnosis, renal cancer cell carcinoma (RCC) is localized to the kidney in 50% of the cases; in another 25%, the cancer will spread to nearby tissue and in the remaining 25% will have metastasized to distal sites. Associated 5-year survival in these patient groups is roughly 90%, 60%, and 9%, respectively. In the United States, the incidence of renal cell carcinoma is higher among African American population. Smoking contributes to one third of all cases of RCC. Other contributing factors are high consumption of fried fatty meat, obesity (particularly in women), and exposure to asbestos and petroleum products. Chemotherapy and radiation are rarely effective for people with renal cancer.
Bladder cancer: Bladder cancer is the 11th most common cancer in the world, with over 50% of the cases found in developed countries[1-4,56]. Smokers have twice the risk of developing bladder cancer than non-smokers. In addition, people exposed to dye, paint, rubber, leather, and hair chemicals are at a greater risk.
We investigated the effect of NM on MMP-2 and MMP-9 secretion in renal cell adenocarcinoma cell line RCC-786-0 and bladder cancer cell line T-24. RCC-786-0 secreted both these enzymes constitutively (table 9,figure 9a,figure 9b). T-24 cells secreted MMP- 2 and MMP-9 only after PMA-induction. The NM inhibited secretion of both MMP-2 and MMP-9 either before or after PMA stimulation with complete suppression occurring at a concentration of 100-500 μg/ml (table 9).
Cancers of the nervous system (glioblastoma, neuroblastoma, retinoblastoma)
Glioblastoma: The most common and most aggressive malignant primary brain tumor is glioblastoma multiforme. Glioblastoma multiforme is a grade IV astrocytoma, a tumor a rising from glial cells surrounding the neurons. Unfortunately, prognosis is very poor and median survival time is approximately 14 months after diagnosis with glioblastoma multiforme [1-4].
Neuroblastoma: It is the most common extra cranial solid cancer in childhood and the most common cancer in infancy, with an annual incidence of about 650 new cases per year in the US. Neuroblastoma is a neuroendocrine tumor arising most frequently occurring in adrenal glands although it can develop in nerve tissues of the neck, chest, abdomen, or pelvis. Prognosis is varied. In very young children, spontaneous remission can occur; in other cases, metastasis to bones (including bone marrow), liver, lymph nodes, and skin is seen. Unfortunately, successful treatment often leaves children at risk for getting a second different kind of cancer in the future.
Retinoblastoma: Retinoblastoma is a cancer of the retina that can occur in hereditary or sporadic forms. Retinoblastoma usually affects children under the age of six, with most diagnoses in children around 1-2 years of age. Prognosis is excellent if metastasis has not occurred. Cure rates of around 95-98% are seen in the developed world.
Melanoma
Melanoma is a skin tumor that originates from the melanocytes and ranks as the seventh leading type of cancer in the US. Its incidenceis ten times higher in Caucasians than in African-Americans. Sun exposure is the principle cause of melanoma. Individuals with fair skin, blue eyes, and blonde or red hair are at highest risk for melanoma. Prognosis varies depending on the stage of the malignancy at the time of diagnosis. For small lesions, surgical resection can be an effective cure. At the other extreme, metastatic malignant melanoma of stage III can have a 5-year survival rate as low as 27% [1-4].
We investigated the effect of NM on melanoma cell line A-2058. In vitro, A-2058 secreted only MMP-2 and after PMA stimulation –the MMP-9 only. Secretion of both enzymes without and after PMA treatment was inhibited by the NM in a dose-dependent manner. Complete suppression of MMP-2 required 1000 μg/ml NM concentration, while MMP-9 suppression was observed at 500 μg/ml of NM (table 11,figure 11a,figure 11b).
Hematologic cancers
Leukemia is a malignancy of bone marrows cells in which clones of immature blood cells multiply at the expense of normal blood cells. As normal blood cells are depleted, anemia, infection, hemorrhage, and eventually death can occur. The peak incidence of Acute Promyelocytic Leukemia (APL) occurs at age of 40 years old whereas T-cell Acute Lymphoblastic Leukemia (T-ALL) between ages 2-5 years. Currently, APL is successfully treated with a combination of vitamin A and the antibiotic anthracycline to achieve a 90% remission rate. T-ALL is currently treated with systemic and CNS chemotherapy, followed by consolidation therapy, totaling 2 years of treatment. Complete remission is seen in approximately 98% of children, and approximately 85% of adults.
We studied the effects of NM on acute promyelocytic leukemia cell line HL-60, acute lymphoblastic T-cell leukemia cell line Jurkat, and Burkitt’s lymphoma cell line Raji. HL-60 secreted MMP-2 constitutively, and MMP-9 only after PMA stimulation. Jurkat (table 12) and Raji (table 12,figure 12a,figure 12b) did not secrete MMP- 2 either constitutively or with PMA stimulation. Jurkat Secretion of MMP-9 was observed in Jurkat cell line after PMA-stimulation, while secretion of MMP-9 occurred constitutively and when stimulated with PMA in Raji cells. In all cell lines tested MMP-2 and MMP-9 secretion was inhibited in a dose-dependent manner after exposure to NM with a complete suppression at a NM concentration of 1000 μg/m (table 12).
Discussion
The aggressiveness of a particular type of cancer is roughly proportional to the rate of tumorigenesis and metastasis [8-21]. The major events of cancer proliferation are cell attachment, angiogenesis to provide the tumor with nutrients, degradation of the ECM to allow tumor expansion, and migration of the tumor cells through the disrupted extracellular matrix [11]. MMPs, particularly the gelatinases MMP-2 and MMP-9, play central roles in these processes due to their ability of to degrade type IV collagen, one of the major components of the basement membranes and ECM. In fact, the aggressiveness of acancer is often directly proportion to the overproduction of MMP-2 and MMP-9 [6-11].In this study we compared the efficacy of a nutrient mixture (NM) consisting of Vitamin C, L-lysine, L-proline, L-arginine, N-acetylcysteine, selenium, copper, manganese, and epigallocatechin gallate (EGCG) on the secretion of MMP-2 and MMP-9 in vitro by 42 different cancer cell lines from representative classes of organ malignancies. The select cancer cell lines studied were grouped by organ malignancies and the MMP-2 and MMP-9 secretion was determined using gelatinase zymography with MMP-2 appearing as a band at 72 kD and MMP-9 as a band at 92 kD. Some cancer cell lines secreted only MMP-2 or MMP-9 and some secreted both.
Our study has shown that NM has an inhibitory effect on MMP-2 and MMP-9 secretion in all 42 cancer lines tested. This effect was dose depended and was observed in every cancer line that secreted MMP- 2 and MMP-9, either constitutively or after PMA stimulation. The effective concentrations of NM required to achieve the total inhibition of MMPs secretion varied between different cancer cell lines. In 34 of the 41 cancer cell lines a suppression of MMP-2 and MMP-9 secretion was achieved at 50 μg/ml-100 μg/ml NM concentrations in the other7 cancer cell lines at 500 μg/ml-1000 μg/ml (table 2-table 12). In the seven cancer lines in which MMP secretion was not completely suppressed by the NM, six of them secreted MMP-9 and only one cell line secreted MMP-2. The secretion of MMP-9 in these six lines was reduced to 1%-2% of control at the maximum NM concentration employed of 1000 μg/ml (table 2 (MCF-7), table 5 (MiaPaCa-2), table 7 (HT- 1080), table 8 (RD), and table 12 (HL-60; Burkitt, Raji)). The secretion of MMP- 2 in thyroid carcinoma was inhibited by 98-99% at a 1000 μg/ml NM (Table 6). Only constitutively secreted MMP-2 in fibrosarcoma line HT-1080 was secreted at 17% of control level at a NM concentration of 1000 μg/ml (table 7). However, the secretion of MMP-2 induced by PMA in this cell line was significantly reduced to 2% of control ata NM concentrati
This study demonstrates the in vitro efficacy of a combination of natural substances (NM) on MMP 2 and MMP-9 secretion in multiple cancer cell lines representing a variety of cancer origins and types. The previous in vivo studies conducted by our group demonstrated modulation of MMP-9 secretion with NM treatment. Tumor tissue from xenograft studies showed reduction of MMP-9 secretion with NM diet [48]. Our other in vivo and vitro studies have documented comprehensive anti-cancer effects of this nutrient combination in over 40 types of cancer cells, in particular in inducing cytotoxicity (table 13), migration, invasion, and metastasis [58] as well as in inhibiting angiogenesis [59,60] and inducing apoptosis[61].
In contrast to toxic side effects of current cancer treatment modalities, the NM is proven to be non-toxic. It has no known side effects on vital organs such as heart, liver, and kidney, when used in the clinically relevant doses as shown in a recent in vivo toxicology study [50]. NM also did not increase the functional serum enzymes indicating that it is safe to use even in high doses far exceeding the normal equivalent doses of the nutrients. Moreover, the NM reduced MMP secretion in vitro and in vivo in xenograft models and reduced chemically induced tumors in our studies [64]. NM is readily absorbed, and is low-cost compared to current cancer treatments. Therefore, we believe NM offers multiple unique benefits in the fight against cancer, worldwide.
References
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, et al. (2008) SEER cancer statistics review (CSR) 1975-2011, (eds), National Cancer Institute.
- http://www.cancer.gov/cancertopics/factsheet/Risk.
- http:/www.cancer.gov/researchandfunding/snapshots.
- Jemal A, Seigal R, Xu J, Ward E (2010) Cancer statistics 2010. CA Cancer J Clin 60: 277-300.
- Fisher JF, Mobashery S (2006) Recent advances in MMP inhibitor design. Cancer Metastasis Rev 25: 115-136.
- Mannello F, Tonti G, Papa S (2005) Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr Cancer Drug Targets 5: 285-298.
- Matter H, Schudok M (2004) Recent advances in the design of matrix metalloproteinase inhibitors. Curr Opin Drug Discov Devel 7: 513-535.
- Wallach J, Hornbeck W (2005) Matrix metalloproteinases and cancer: Novel perspectives in the role and control of matrix metalloproteinases in tumor progression. Biochimie 87: 241.
- 9. Fingleton B (2006) Matrix metalloproteinases. Roles in cancer and mestastasis. Front Biosci 11: 479-491.
- 10. Demers M, Couillard J, Belanger S, St Pierre Y (2005) New roles for matrix metalloproteinases in mestastasis. Crit Rev Immunol 25: 493-523.
- Fidler IJ (1997) Molecular biology of cancer: invasion and metastasis in cancer. Principles and practice of Oncology, (eds), Lippincott-Raven, Philadelphia pp: 135-152.
- Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161-174.
- Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29: S15-S18.
- Chambers AF, Matrisian LM (1997) Changing views on the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89: 1260-1270.
- Kleainer DL, Stetler-Stevenson WG (1999) Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 43: S42-S51.
- Barsky SH, Siegel GP, Jannotta F, Liotta LA (1983) Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest 49: 140-147.
- Liotta LA, Tryggvason K, Garbisa A, Hart I, Foltz CM, et al. (1980) Metatstatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284: 67-68.
- Nelson AR, Fingleton B, Rothenberg, ML, Matrisian LM (2000) Matrix metalloproteinases:biologic activity and clinical implications. J Clin Oncol 18: 1135-1149.
- Stetler-Stevenson WG (2001) The role of matrix metalloproteinases in tumor invasion, metastasis, and angiogenesis. Surg Oncol Clin N Am 10: 383-392.
- Stetler-Stevenson WG (1990) Type IV collagenases in tumor invasion, metastasis. Cancer Metastasis Rev 9: 289-303.
- Nemeth JA, Yousif R, Herzog M, Che M, Upadhyay J, et al. (2002) Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst 94: 1-25.
- Kemberling JK, Hampton JA, Keck RW, Gomez MA, Selman SH (2003) Inhibition of bladder tumor growth by the green tea derivative epigallocatechin-3-gallate. J Urol 170: 773-776.
- Valcic S, Timmermann BN, Alberts DS, Wachter GA, Krutzsch M, et al. (1996) Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anticaner Drugs 7: 461-468.
- Sato D, Matsushima M (2003) Preventive effects of urinary bladder tumors induced by N-butyul-N-(4-hydroxybutyl)-nitrosamine in rat by green tea leaves. Int J Urol 10: 160-166.
- Yang GY, Liao J, Kim K, Yurtow EJ, Yang CS (1998) Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 19: 611-616.
- Taniguchi S, Fujiki H, Kobayashi H, Go H, Miyado K, et al. (1992) Effect of (-)-epigallocatechin, the main component of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett 65: 51-54.
- Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M (2005) In vivo antitumor effect of ascorbic acid, lysine, proline and green tea extract on human prostate cancer PC-3 xenografts in nude mice: evaluation of tumor growth and immunhohistochemistry. In Vivo 19: 179-183.
- Sun Z, Chen YH, Wang P, Zhang J, Gurewich V, et al. (2002) The blockage of high-affinity lysine binding sites of plasminogen by EACA significantly inhibits prourokinase-induced plasminogen activation. Biochem Biophys Acta 1596: 182-192.
- Rath M, Pauling L (1996) Plasmin-induced proteolysis and the role of apoprotein(a), lysine and synthetic analogs. Orthomol Med 7: 17-23.
- Kawakami S, Kageyama Y, Fujii Y, Kihara K, Oshima H (2001) Inhibitory effects of N-acetyl cysteine on invasion and MMP-9 production of T24 human bladder cells. Anticancer Res 21: 213-219.
- Morini M, Cai T, Aluigi MG, Noonan DM, Masiello L, et al. (1999) The role of the thiol N-acetyl cysteine in the prevention of tumor invasion and angiogenesis. Int J Biol Markers 14: 268-271.
- Nunez C, Ortiz de Apodaca Y, Ruiz A (1995) Ascorbic acid in the plasma and blood cells of women with breast cancer: The effect of consumption of food with an elevated content of this vitamin. Nutr Hosp 10: 368-372.
- Kurbacher CM, Wagner U, Kolster B, Andreotti PE, Krebs D, et al. (1996) Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett 103: 183-189.
- Maramag C, Menon M, Balaji KC, Reddy PG, Laxmanan S (1997) Effect of vitamin C on prostate cancer cells in vitro: effect on cell number, viability and DNA synthesis. Prostate 32: 185-195.
- Koh WS, Lee SJ, Lee H, Park C, Park MH, et al. (1998) Differential effects and transport kinetics of ascorbate derivatives in leukemic cell lines. Anticancer Res 8: 2487-2493.
- Naidu KA, Karl RC, Coppola D (2003) Antiproliferative and proapoptotic effect of ascorbyl stearate in human pancreatic cancer cells: association with decreased expression of insulin-like growth factor 1 receptor. Dig Dis Sci 48: 230-237.
- Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, et al. (2005) Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A 102: 13604-13609.
- Yeom CH, Lee G, Park JH, Yu J, Park S, et al. (2009) High-dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via restriction of angiogenesis. J Transl Med 7: 70.
- Anthony HM, Schorah CJ (1982) Severe hypovitaminosis C in lung-cancer patients: utilization of vitamin C in surgical repair and lymphocyte-related host resistance. Br J Cancer 46: 354-367.
- Hara Y (2003) Green tea: health benefits and applications book. Am J Clin Nutr 77: 754-755.
- Chen D, Daniel KG, Kuhn DJ, Kazi A, Bhuiyan M, et al. (2004) Green tea and tea polyphenols in cancer prevention. Front Biosci 9: 2618-2631
- Schrauzer GN (2000) Anticarcinogenic effects of selenium. Cell Mol Life Sci 57: 1864-1873.
- Yoon SO, Kim MM, Chung AS (2001) Inhibitory effects of selenite on invasion of HT-1080 tumor cells. J Biol Chem 276: 20085-20092.
- Rayman MP (2005) Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc 64: 527-542.
- Cooke JP, Dzau VJ (1997) Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med 48: 489-509.
- Netke SP, Roomi MW, Roomi NW, Ivanov V, Niedzwiecki A, et al. (2003) A specific combination of ascorbic acid, lysine, proline and epigallocatechin gallate inhibits proliferation and extracellular matrix invasion of various human cancer cell lines. Res Commun Pharmacol Toxicol Emerg Drugs 2: 37-50.
- Roomi MW, Roomi N, Ivanov V, Kalinovsky T, Niedzwiecki A, et al. (2005) Inhibitory effect of a mixture containing ascorbic acid, lysine, proline and green tea extract on critical parameters in angiogenesis. Oncol Rep 14: 807-815.
- Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M, et al. (2006) Inhibition pulmonary metastasis of melanoma B16FO cells in C57BL/6 mice by a nutrient mixture consisting of ascorbic acid, lysine, proline, arginine and green tea. Exp Lung Res 32: 517-530.
- Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M (2006) In vivo and in vitro anti-tumor effect of ascorbic acid, lysine, proline, arginine and green tea extract on human fibrosacrcoma cells HT-1080. Med Oncol 23: 105-111.
- Roomi MW, Kalinovsky T, Monterrey J, Rath M, Niedzwiecki A, et al. (2008) A nutrient mixture suppresses hepatic metastasis in athymic nude mice injected with murine B16FO melanoma cells. Biofactors 33: 181-189.
- Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M (2010) Micronutrient synergy-a new tool in effective control of metastasis and other key mechanisms of cancer. Cancer Metastasis Rev 29: 529-542.
- Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A (2010) Comparative effects of EGCG, green tea and a nutrient mixture on the patterns of MMP-2 and MMP-9 expression in cancer cell lines. Oncol Rep 24: 747-757.
- Rahul Amin ARM, Kucuk O, Khuri FR, Shin DM (2009) Perspectives for cancer prevention with natural compounds. J Clin Oncol 27: 2712-2725.
- Roomi MW, Ivanov V, Netke SP, Niedzsiecki, Rath M (2003) Serum markers of the liver, heart, and kidney and lipid profile and histopathology of ODS rats treated with nutrient synergy. J Am Coll Nutr 22: 77.
- Widakowich C, de Azambula E, Gil T, Dionh P, Awada A, et al. (2007) Molecular targeted therapies in breast cancer: where are we now? Int J Biochem Cell Biol 39: 1375-1387
- Wicha MS, Liu S, Dontu G (2006) Cancer stem cells: an old idea-a paradigm shift, Cancer Res 66: 1883-1890.
- Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI (2007) Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25: 5165-5171.
- Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, et al. (2007) Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 25: 526-531.
- Kenny PM, King MT, Viney RC, Boyer MJ, Pollicino CA, et al. (2008) Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol 26: 233-241.
- von der Maase H (2002) Current and future perspectives in advanced bladder cancer: is there a new standard? Semin Oncol 29: 3-14.
- Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A (2010) Inhibition of invasion and mmps by a nutrient mixture in human cancer cell lines: a correlation study. Exp Oncol 32: 243-248
- Roomi MW, Roomi N, Ivanov V, Kalinovsky T, Niedzwiecki A, et al. (2005) Inhibitory effect of a mixture containing ascorbic acid, lysine, proline, and green tea extract on critical parameters in angiogenesis. Oncology Reports 14: 807-815
- Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M (2009) Antiangiogenic properties of a nutrient mixture in a model of hemangioma. Exp Oncology 31: 214-219.
- Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M (2005) Modulation of N-Methyl –N-nitrosourea-induced mammary tumors in sprague-dawley rats by combination of lysine, proline, arginine, ascorbic acid and green tea extract. Breast Cancer Research 7: R291-R295.