Journal of Obesity and Bariatrics
Download PDF
Research Article
Portomesentric Vein Thrombosis: Our Experience of 3527 Laparoscopic Bariatric Surgery Procedures in Qatar
Moamena Ahmed El-Matbouly1*, Nesreen Khidir2, Davit Sargsyan1, Walid El Ansari3, Moataz Bashah1 and Mohammed Al-Kuwari1
- 1Department of General Surgery, Hamad Medical Corporation, Doha, Qatar
- 2Department of bariatric and metabolic Surgery, Hamad Medical Corporation, Doha, Qatar
- 3Department of Surgery, Hamad Medical Corporation, Doha, Qatar
*Address for Correspondence: Moamena Ahmed El-Matbouly, Department of General Surgery, Hamad Medical Corporation, Doha, Qatar, Tel: +974-55910445; E-mail: momenaelmatbouly@gmail.com
Citation: El-Matbouly MA, Khidir N, Sargsyan D, El Ansari W, Bashah M, et al. Portomesentric Vein Thrombosis: Our Experience of 3527 Laparoscopic Bariatric Surgery Procedures in Qatar. J Obes Bariatrics. 2018;5(1): 6.
Copyright: © 2018 El-Matbouly MA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Obesity and Bariatrics | ISSN: 2377-9284 | Volume: 5, Issue: 1
Submission: 19 April, 2018 | Accepted: 21 May, 2018 | Published: 31 May, 2018
Keywords
Portomesenteric vein thrombosis; Bariatric complications; Hypercoagluabitlity; Laparoscopic bariatric surgery
Abstract
Background: Portomesentric vein thrombosis (PMVT) is a rare but potentially serious and life threatening complication that may happen following laparoscopic bariatric surgery (LBS). Multifactorial local and systemic prothrombotic factors have been identitfied for PMVT development. In this study we intend to assess: 1) Incidence of PMVT post LBS in our patients’ population; 2) Our protocol for DVT prophylaxis with applying the enhanced recovery after surgery (ERAS) protocol and application of certain intra-operative techniques in PMVT prevention following LBS.
Methods: This is a retrospcetive review of patients who underwent LBS between February 2011 and December 2016, who developed PMVT following LBS. Patients’ charts were reviewed and data were extracted (patients’ demographics, associated comorbidities, potential PMVT risk factors, LBS procedure undertaken, peri-operative details). In addition, the clinical features were also retrieved (PMVT presenting symptoms, management, blood investigations and thrombophilia screening).
Results: Out of 3527 patients who underwent LBS in our center during this period, 4 patients developed PMVT comprising an incidence of 0.1%. Thrombophilic work up of patients who developed PMVT revealed that all had thrombosis risk factors (hyper-homocysteinema, anti-nuclear antibody, SLE, mutant Factor V Leiden, Protein C, Protein S deficiency). All managed medically and none required surgical intervention. There was no mortality.
Conclusion: Though no consensus concerning PMVT prophylaxis in LBS patients exists, we propose that application of a standard multimodal thromboembolic prophylaxis mainly mechanical prophylaxis, intra-operative techniques and ERAS principles as showed, were major reasons for low PMVT incidence.
All patient who developed PMVT post LBS had positive diagnostic throbophilia work up. Following standardized non-pharmacological thromboembolic prophylaxis in LBS patients questions the benefit of pharmacological thromboembolic prophylaxis.
Abbrevations
PVMT: Portomesenteric Vein Thrombosis; LBS: Laparoscopic Bariatric Surgery; LRYGB: Laparoscopic Roux-en-Y Gastric Bypass; GERD: Gastroesophageal Reflux Disease; OA: Osteoarthritis; LMWH: Low-Molecular-Weight Heparin; IAP: Intra-Abdominal Pressure; OR: Operation Room; LOS: Length of Hospital Stay; DVT: Deep Venous Thrombosis; PE: Pulmonary Embolism; UFH: Unfractionated Heparin; SMV: Superior Mesenteric Vein; VTE: Venous thromboembolism; IVC: Inferior Vena Cava; MVT: Mesenteric Venous Thrombosis; EGD: Esophagogastroduodenoscopy
Background
Thrombosis of portal vein and mesentric vein (Porto Mesenteric Venous Thrombosis, PMVT) is fortunatley a relatively uncommon complication in laparascopic bariatric surgery (LBS) patients [1]. However, PMVT requires prompt attention as delayed diagnosis could have potentially life-threatening consequences. For instance, PMVT accounts for 5-15% of reported cases of acute mesenteric ischemia, which could progress to bowel necrosis and sepsis, ultimately leading to death [2]. PMVT has been observed after surgical procedures, but the inflammatory and hypercoagulable obese state, laparoscopy, and manipulation of the portomesenteric venous system contribute to the increased risk of PMVT after LBS [3,4].
PMVT post laparoscopic Roux-en-Y gastric bypass (LRYGB) was first reported in 2004 and post laparoscopic sleeve gastrectomy (LSG) in 2009 [5]. Diagnosing PMVT is challenging in the obese patients post LBS period, with vague and non-specific symptoms and complaints [6]. Multiple diagnostic modalities are usually employed to establish the diagnosis e.g. CT scan, doppler ultrasound and MRI [7]. Currently with the advancement in testing for hypercoagulable states, patients diagnosed with PMVT can undergo additional investigations to identify possible predisposing risk factors to thrombus formation. Such risk factors include factor V Leiden mutation, prothrombin G20210A mutation, protein S deficiency, protein C deficiency, antithrombin-III deficiency, activated protein C resistance and antiphospholipid syndrome which have all been linked to increased splanchnic thrombosis [8].
Review of the literature revealed a limited number of case reports and series describing PMVT following laparoscopic bariatric surgery as an uncommon complication [4]. A retrospective anaylsis of 1713 patients who have undergone LSG showed an incidence of 1% PMVT [1]. In our study we present the cases of PMVT following LBS performed at our Metabolic and Bariatric Surgery Center to assess our incidence of PMVT post LBS; the patient and the procedure-related thrombosis risk factors; and the effect of employing the multimodal thromboembolism prophylaxis, ERAS protocol, as well as application of certain intra-operative techniques on PMVT incidence post-LBS.
Materials and Methods
A retrospective review of all patients who underwent elective LBS in our center between February 2011-December 2016 and subsequently developed symptomatic PMVT.
For each identified PMVT patient, the following variables were extracted from the records: age, gender, initial body mass index (BMI), risk factors for thrombosis (patient history, family history, and medication history), surgical technique used and its details, PMVT presenting clinical features and management. The mean follow up duration was 12 months for the entire study group. For the patients who were diagnosed with PMVT they were followed for three months initially and then at 6 months.
Application of multimodal thromboembolic prophylaxis, ERAS principles and certain intra-operative techniques
In our practice, the ERAS protocol includes peri-operative and post-operative techniques like; mechanical thromboembolic prophylaxis consisting of peri-operative compression stockings and early post-operative ambulation.
Pharmacological thromboembolism prophylaxis: unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) was limited in our practice to the high risk group (according to our assessment), e.g. those with BMI>50, bed or wheelchair bound patients, with history of previous thromboembolic events or known familial thrombophilic diseases and patients on hormonal or contraceptive medications. Those selected patients received LMWH (dalteparin sodium injection 5000 IU) started 6 hours post-operatively and continued to a total of 14 days after discharge.
All patients who presented with symptomatic PMVT were closely monitored and observed clinically (symptoms, signs and blood works) and if needed radiologically. CT, U/S and Thrombophilia workup was conducted for all PMVT patients as well as follow up CT scan on outpatient basis. A follow up upper gastrointestinal endoscopy (UGE) was performed in all PMVT patients at 1 year post diagnosis, to identify signs of portal hypertension.
Results
Among the 3527 patients who underwent LBS, mean BMI was 44.2±8.8, 5 kg/m2 with 99% (CI 43.8-44.6). Pharmacological thromboembolism prophylaxis was given to 627 (19%) patients; 97% of these patients had BMI>50, and 19 (3%) patients had venous thrombosis risk factors; known family or personal history of DVT, bed or wheelchair bound and patients on hormonal contraceptive therapy. While 81% of our LBS patients did not receive pharmacological thromboembolism prophylaxis. Four of these pateints (0.1%) developed PMVT and 25 (0.9%) developed post-op bleeding that required laparoscopic intervention and blood transfusion.
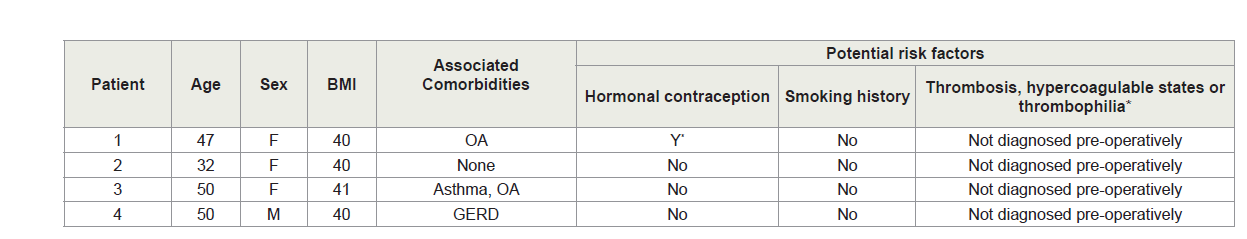
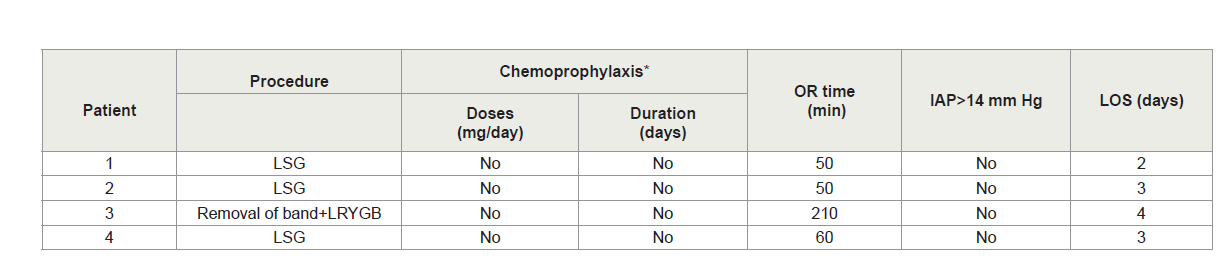
The PMVT patients’ mean age was 44.8 (±8.6 years) and three patients were women. The average BMI was 40.3 (±0.5 kg/m2). PMVT Patients’ characteristics are summarized in Table 1. None of the PMVT patients received pre or post-op pharmacological thromboembolism prophylaxis. Mean length of surgery was 92.5 (±78.5 min). No intra-operative or immediate post-operative complications occurred. The peri-operative details for the PMVT patients including intraoperative and post-operative course are summarized in Table 2. Patients’ mean length of hospital stay was 3±0.8 SD days.
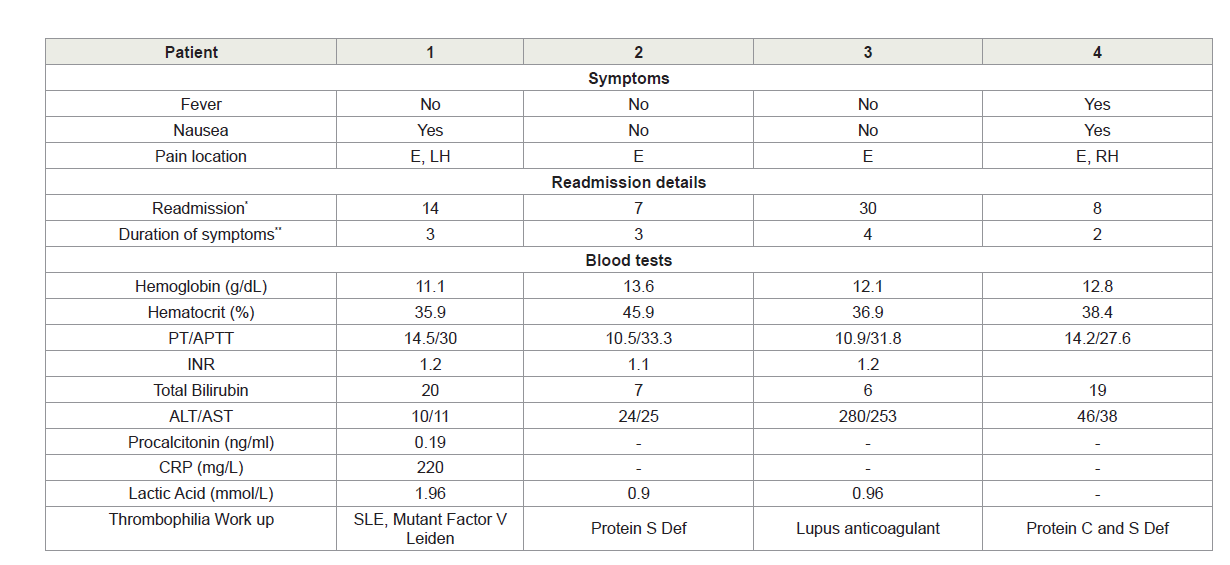
All PMVT cases started to have symptoms after discharge, with a mean duration of symptoms of 3 days (range 2-4 days). Patients were re-admitted after M=17 days (range 5-30 days) post their initial surgery, The PMVT readmission characteristics are shown in Table 3. All the patients had chief complaint of vague abdominal pain mainly in the epigastrium with nausea. All cases were diagnosed by abdominal and pelvic CT enhanced with oral and intravenous contrast. One patient had partial thrombosis in the superior mesenteric vein (SMV) only, while the remaining three had partial thrombosis in the SMV and portal vein. Patients were then subsequently assessed for bowel ischemia and/or signs of sepsis and none of them required surgical intervention. All the four patients had successful medical management for their PMVT (anti-coagulation, low molecular weight heparin and warfarin). None of the patients required percutaneous trans-hepatic thrombolytic therapy.
Discussion
All patients undergoing bariatric surgery BMI≥35 are considered of moderate to high risk of post-operative PMVT, deep venous thrombosis (DVT) and pulmonary embolism (PE) [9,10].
The occurrence of PMVT post LBS may be attributed to the following factors:
1. Hypercoagulable state associated with obesity;
2. Injury to the porto-mesenteric vessels; mechanical or thermal effect during dissection of the greater curvature on the left gastroepiploic and short gastric vessels, direct injury with the splenic or superior mesenteric vein (SMV);
3. The inflammatory response to surgery;
4. Laparoscopy causing a thrombogenic effect due to increased intraabdominal pressure with reduction in venous return from the extremities (especially in the prolonged reverse Trendelenburg position used in LBS) and splanchnic system;
5. Blood stasis within the liver caused by prolonged positioning of the liver retractor, leading to formation of a retrograde thrombosis is also proposed;
Currently, there are no available accurate evidence-based risk assessment tools for VTE in bariatric patients. The published literature varies widely on optimal guidelines for the prevention of VTE following LBS. The major accepted forms of prophylaxis range from mechanical compression devices and early ambulation alone, to the addition of chemoprophylaxis and the use of IVC filters [11].
Becattini C et al. mentioned a 2% incidence of bleeding complications associated with chemoprophylaxis that raised concerns regarding administering anticoagulation post operatively [12]. Several studies have examined the use of mechanical compression only in bariatric surgery patients [12]. A retrospective study of 1692 patients reported DVT and PE rates of 1.6% and 1.1% respectively, in patients who received LMWH and the sequential compression devices compared with a 0.4% DVT rate and no PEs in the patients who receive mechanical prophylaxis and early ambulation only. The incidence of PMVT was not reported in the study [11]. The incidence of post-operative VTE complications, either symptomatic or asymptomatic seems to be relatively low <1%, and the benefit of weight-adjusted heparin prophylaxis remains controversial. The incidence of symptomatic VTE in the 2 large studies with mechanical prophylaxis appeared to be similar to that observed with heparin prophylaxis [12].
In this study we present our incidence of PMVT over LBS population who received only mechanical prophylaxis. We stress on the same point by Becattini C et al. who suggested that mechanical prophylaxis in addition to the application of enhanced recovery after surgery is sufficient for patients without personal or strong family history of VTE events or known hypercoagulable state [12]. In order to prevent blood stasis peri-operatively, we systematically use a valveless trocar system to control intra-abdominal pressure since a 50% reduction in portal blood flow has been demonstrated when intra-abdominal pressure exceeds 14 mm Hg [13]. We suggest that the collective application of these techniques comprise major contributing factors in reducing the operative time, venous stasis and blood re-distribution in the splanchnic circulation leading to the decreased PMVT incidence that we observed among our LBS population.
We determined our incidence of PMVT post LBS among 3551 various elective LBS procedures’ to be (incidence=0.1%). Few published studies are available on the topic with which we can directly contrast our findings. A research by Salinas and others, resulted in 1% PMVT incidence following 1713 LSG procedures [1]; and the lowest published PMVT incidence after LBS was 0.3% [10]. It should also be noted that the PMVT cases reported here were based on symptomatic patients who underwent diagnostic imaging and no routine imaging or screening was performed for asymptomatic patients.
Regarding patient-related predisposing PMVT risk factors; all our PMVT had positive thrombophilia workup. This finding contrasts with others who reported that out of 17 patients who developed post-LBS PMVT, only four patients exhibited ≥1 of these risk factor/s [10]. In a recent review, 8 out of 36 cases of post LBS-PMVT had a positive thrombophilia workup [14]. The four patients who had PMVT had missed family history that was positive for thrombophilia.
Regarding procedure-related predisposing PMVT risk factors, we observed that three of our four PMVT patients were post LSG, in support of a very recent review that found that the majority of PMVT cases were after LSG, suggesting that it is procedure specific [14]. Others reported that of 5706 LBS patients, 17 (0.3%) had PMVT, 16 of which were post-LSG [10]. Many propositions have been put forward for the high PMVT rate after LSG making it procedure specific. For instance, the intra-operative manipulation of the splanchnic vessels along the stomach’s greater curvature as well as the thermal injury to the short gastric vessels when sealed with energy devices [14].
The current literature has evaluated the effectiveness of ERAS principles in improving post operative recovery and in-hospital stay in LBS [15]. To the best of our knowledge, there seems no exisiting evaluations of the impact of the use ERAS principles on post operative morbidity and mortality in LBS [15]. An exception is Grantcharov H et al. who examined the value of an accelerated recovery program in elective primary laparoscopic gastric resection [16]. Their prospective study demonstrated that the successfull clinical application of ERAS principles led to decrease in post-operative complications [16]. The current study reports the effect of application ERAS principles as a major contribution in achieving a low rate of post-operative incidence of PMVT.
Our study is an observational retrospective study that looked at the patients who had LBS and we are reporting our incidence of PMVT as well as the protocol that we used for DVT prophylaxis. One of the limitations of the study is the lack of comparison between mechanical and pharmachological prophylaxis. In our study we are reporting our finding of observing our population post LBS and stating the protocol that we followed in our institution. Future studies should be done to look into the effectivness of pharmachological DVT prophylaxis and its impact on the incidence of PMVT post LBS.
Diagnosis and treatment
The clinical diagnosis of PMVT is challenging post LBS. High index of suspicion is required in patients presented with vague abdominal pain post operatively. Contrast CT abdomen has a sensitivity of 95% [17].
Close observation and frequent monitoring of these patients are mandatory to avoid unnecessary surgical interventions considering a high index of suspicion of thrombosis progressing to mesenteric ischemia, sepsis and bowel infarction. Any delay in diagnosing intestinal infarction worsens the prognosis; thus, making early diagnosis a key factor in improving the patient’s outcome. In our practice once a suspicion of bowel ischemia or sepsis is raised, we consider measuring sepsis biomarkers; procalcitonin, CRP and lactic acid as effective tools to guide our clinical and therapeutic decisions. Procalcitonin is more sensitive and specific diagnostic parameter for sepsis and a better predictor of mortality [18].
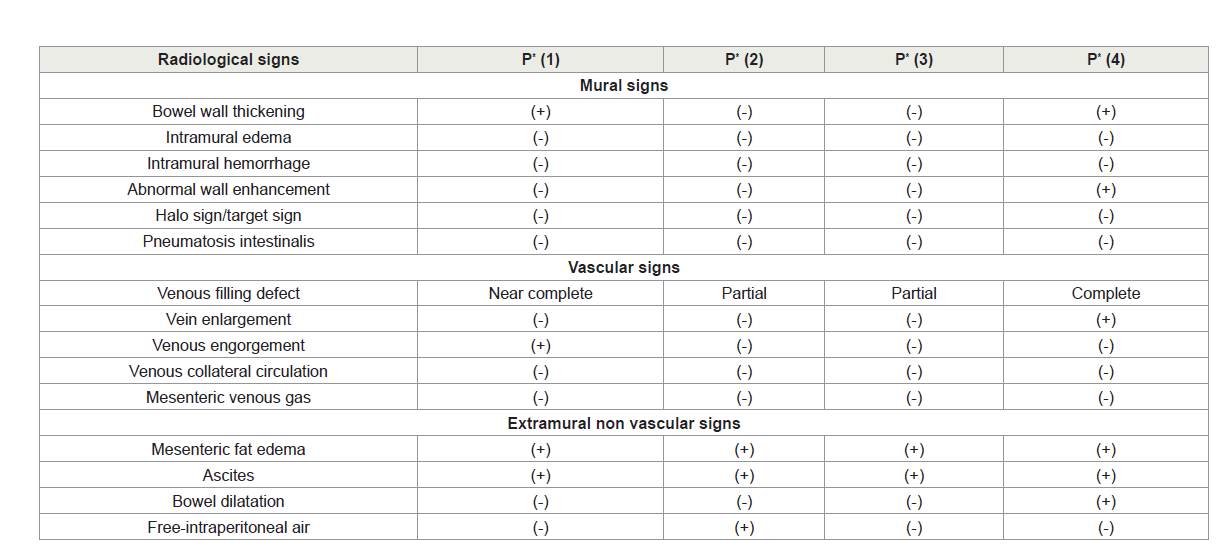
In this article, we provide an overview of the diverse radiological signs of CT imaging, as an important factor in predicting the occurrence of subsequent bowel ischemia and infarction. Subdividing them into categories facilitates comprehension; and decision making regarding progression to the operating room and exploration. Initial CT findings of our PMVT patients are shown in Table 4. Sometimes serial CT imaging was required [17].
The radiological signs of bowel ischemia can be divided into mural, vascular, and extramural signs. Circumferential bowel wall thickening, caused by intramural hemorrhage and edema is the most commonly reported sign in acute venous bowel ischemia. It is more pronounced with venous congestion due to mesenteric venous thrombosis (MVT) than with arterial thrombosis. Decreased bowel wall enhancement has been reported as highly specific for bowel infarction in patients with MVT [17]. Signs of mucosal edema-“halo sign” (mural stratification into two layers). Stratification into three layers is also called the “target sign”), which is composed of three rings: outer high-attenuation muscularis propria, a middle ring of gray attenuation, and a inner ring of high attenuation (mucosa) [19]. Venous filling defect may be partial or complete. Vein caliber enlargement is an indicative of an acute thrombosis. Extramural non vascular signs such as pneumatosis intestinalis and portomesenteric venous gas, when present transmural bowel infarction was seen in 91% of patients [19]. Mesenteric fat edema is related to the underlying inflammatory processes. Bowel dilatation results from interrupted intestinal peristalsis in response to ischemic injury and bowel infarction. Free intraperitoneal air results from perforation of an infarcted bowel segment. Presence of free intraperitoneal air in our patient number 2, was correlated to the retained CO2 used for pneumoperitoneum; CT was done on day 7 post surgery.
We stress on the point that mural and extramural-nonvascular CT signs of bowel ischemia are nonspecific. However, if CT findings are suspicious and not conclusive or if the patient shows signs of deterioration into sepsis, laparoscopic exploration is highly indicated.
Once PMVT is diagnosed; full anticoagulation with either subcutaneous LMWH or intravenous unfractionated heparin was applied. This treatment was continued and changed to oral anticoagulation (target international normalized ratio, 2.5-3), which continued for 6 months. Early diagnosis and treatment with prompt anticoagulant therapy could lead to a dramatic decrease in the incidence of prehepatic portal hypertension in the near future. In contrast, late detection and treatment may result in prehepatic portal hypertension with the associated sequelae and portal cavernomatosis; resulting in portal hypertension. A screening esophagogastroduodenoscopy (EGD) for esophageal varices was done for post-PMVT patients at one year and was negative for all patients.
Conclusion
Although there is no consensus concerning PMVT prophylaxis in LBS patients, we propose that application of a standard multimodal thromboembolism prophylaxis, intra-operative techniques and ERAS principles as described, were major reasons for low PMVT incidence in our series.
All the patient who developed PMVT post LBS had positive diagnostic throbophilia work up. Following standardized non-pharmacological thromboembolic prophylaxis in LBS patients questions the benefit of pharmacological thromboembolic prophylaxis. Careful clinical monitoring of the patient and proper comprehension of the CT signs of bowel ischemia can avoid further aggressive interventions and exploration. Proper comparative study between mechanical and pharmacological DVT prophylaxis should be done to assess its impact on the incidence of PMVT post LBS.
Acknowledgement
We would like to thank Mr. Arnel Alviz, system analyst for helping in collecting the data and gathering the crude information for the patients.
References
- Salinas J, Barros D, Salgado N, Viscido G, Funke R, et al. (2014) Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy. Surg Endosc 28: 1083-1089.
- Keung CH, Gander JW, Zitsman JL (2015) Mesenteric venous thrombosis following vertical sleeve gastrectomy in an adolescent. Surg Obes Relat Dis 11: e23-e26.
- Bhatia P, John SJ, Kalhan S, Bindal V (2015) Portomesenteric venous thrombosis after laparoscopic sleeve gastrectomy: a case report and a call for prevention. J Minim Access Surg 11: 276-278.
- Villagrán R, Smith G, Rodriguez W, Flores C, Cariaga M, et al. (2016) Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy: incidence, analysis and follow-up in 1236 consecutive cases. Obes Surg 26: 2555-2561.
- Berthet B, Bollon E, Valero R, Ouaissi M, Sielezneff I, et al. (2009) Portal vein thrombosis due to factor 2 leiden in the post-operative course of a laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg 19: 1464-1467.
- Muneer M, Abdelrahman H, El-Menyar A, Zarour A, Awad A, et al. (2016) Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy: 3 case reports and a literature review. Am J Case Rep 17: 241-247.
- Hmoud B, Singal AK, Kamath PS (2014) Mesenteric venous thrombosis. J Clin Exp Hepatol 4: 257-263.
- Riva N, Donadini MP, Dentali F, Squizzato A, Ageno W (2012) Clinical approach to the splanchnic vein thrombosis: risk factors and treatment. Thromb Res 130 Suppl 1: S1-S3.
- Rocha AT, de Vasconcellos ÂG, da Luz Neto ER, Araújo DM, Alves ES, et al. (2006) Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg 16: 1645-1655.
- Goitein D, Matter I, Raziel A, Keidar A, Hazzan D, et al. (2013) Portomesenteric thrombosis following laparoscopic bariatric surgery: incidence, patterns of clinical presentation, and etiology in a bariatric patient population. JAMA Surg 148: 340-346.
- American Society for Metabolic and Bariatric Surgery Clinical Issues Committee (2013) ASMBS updated position statement on prophylactic measures to reduce the risk of venous thromboembolism in bariatric surgery patients. Surg Obes Relat Dis 9: 493-497.
- Becattini C, Agnelli G, Manina G, Noya G, Rondelli F (2012) Venous thromboembolism after laparoscopic bariatric surgery for morbid obesity: clinical burden and prevention. Surg Obes Relat Dis 8: 108-115.
- Herati AS, Andonian S, Rais-Bahrami S, Atalla MA, Srinivasan AK, et al. (2011) Use of the valveless trocar system reduces carbon dioxide absorption during laparoscopy when compared with standard trocars. Urology 77: 1126-1132.
- El Lakis MA, Pozzi A, Chamieh J, Safadi B (2017) Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass: a 36-case series. Surg Endosc 31: 1005-1011.
- Lemanu DP, Singh PP, Berridge K, Burr M, Birch C, et al. (2013) Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg 100: 482-489.
- Grantcharov TP, Kehlet H (2010) Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg 97: 1547-1551.
- Lee SS, Ha HK, Park SH, Choi EK, Kim AY, et al. (2008) Usefulness of computed tomography in differentiating transmural infarction from nontransmural ischemia of the small intestine in patients with acute mesenteric venous thrombosis. J Comput Assist Tomogr 32: 730-737.
- Shiferaw B, Bekele E, Kumar K, Boutin A, Frieri M (2016) The role of procalcitonin as a biomarker in sepsis. J Infect Dis Epidemiol 2: 006.
- Wittenberg J, Harisinghani MG, Jhaveri K, Varghese J, Mueller PR (2002) Algorithmic approach to CT diagnosis of the abnormal bowel wall. Radiographics 22: 1093-1109.