Journal of Obesity and Bariatrics
Download PDF
Research Article
*Address for Correspondence: Yishai Levy, MD, Department Medicine, Rambam Health CareCampus, Bat Galim, Haifa, Israel, Tel: 972-4-7772263; Fax: 972-4-7773286; E-mail: ys_levy@rambam.health.gov.il
Citation: Levy Y, Dabbah I, Mahajna A, Gaitini D, Assalia A. Bariatric Surgery for the Treatment of Morbid Obesity: Early effects on Glycemic Control, Inflammatory and Carotid Arteries Wall Thickness Status. J Obes Bariatrics. 2015;2(1): 5.
Copyright © 2015 Levy Y, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Obesity and Bariatrics | ISSN: 2377-9284 | Volume: 2, Issue: 1
Submission: 30 March 2015| Accepted: 15 April 2015 | Published: 20 April 2015
Bariatric Surgery for the Treatment of Morbid Obesity: Early effects on Glycemic Control, Inflammatory and Carotid Arteries Wall Thickness Status
Yishai Levy1,2*, Ihab Dabbah1,2, Ahmad Mahajna2,3, Diana Gaitini2,4 and Ahmad Assalia2,3
- 1Department of Medicine, Rambam Health Care Campus, Bat Galim, Haifa, Israel
- 2Rappaport Faculty of Medicine, Technion, Israel, Institute of Technology, Bat Galim, Haifa, Israel
- 3Department of General Surgery, Rambam Health Care Campus, Bat Galim, Haifa, Israel
- 4Department of Medical Imaging, Unit of Ultrasound, Rambam Health Care Campus, Haifa, Israel
*Address for Correspondence: Yishai Levy, MD, Department Medicine, Rambam Health CareCampus, Bat Galim, Haifa, Israel, Tel: 972-4-7772263; Fax: 972-4-7773286; E-mail: ys_levy@rambam.health.gov.il
Citation: Levy Y, Dabbah I, Mahajna A, Gaitini D, Assalia A. Bariatric Surgery for the Treatment of Morbid Obesity: Early effects on Glycemic Control, Inflammatory and Carotid Arteries Wall Thickness Status. J Obes Bariatrics. 2015;2(1): 5.
Copyright © 2015 Levy Y, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Obesity and Bariatrics | ISSN: 2377-9284 | Volume: 2, Issue: 1
Submission: 30 March 2015| Accepted: 15 April 2015 | Published: 20 April 2015
Abstract
Background: Cardiovascular diseases and type 2 diabetes mellitus (T2DM) are the leading reasons for premature morbidity and mortality in patients with extreme obesity. Currently, there is limited success in changing this course by nutritional and pharmacological intervention. Therefore, we have attempted to assess the early effects of bariatric surgery on metabolic, glycemic, inflammatory and on carotid arteries wall thickness status in obese patients, all of them mediate accelerated atherosclerotic in these patients.Methods: Twenty two patients, 11 with and 11 without T2DM were followed for three months after surgery. Fasting blood glucose, HbA1C and lipids were performed and carotid intima-media thickness (IMT) was measured.
Results: Bariatric surgery resulted in a significant weight loss from 126±24 to 102±21 kg (mean±SD, p<0.0001) with a 46±13% reduction in excess weight (EWL). Some markers of inflammation dropped significantly: White blood cells (WBC) from 9.3x103 to 7.6x103/μL and C-Reactive Protein (CRP) from 13.5 to 8.2 mg/L (p<0.0001). Fasting glucose decreased from 126 to 102 mg/dL (p=0.006) while HbA1C decreased from 7.2% to 5.7% (p<0.0001). In patients with diabetes, fasting glucose dropped from 162 to 122 mg/dL(p=0.04) whereas HbA1C dropped from 8.2 to 5.9% (p=0.005), thus moving from a diabetic to a non-diabetic range. IMT did not show any improvement throughout the 3 month study.
Conclusion: Bariatric surgery had dramatic effects on glycemic control and inflammatory status in patients with T2DM as early as three months after surgery. If sustained, such response after surgery may confer protection against future cardiovascular morbidity and mortality through mechanisms presented in our study.
Keywords
Bariatric surgery; Blood lipids; C-reactive protein (CRP); Diabetes mellitus; Intima media thickness (IMT); Morbid obesityIntroduction
Obesity is characterized by excess body fat mass. Although it is not an accurate measure of body fat, Body Mass Index (BMI) measured as weight/height2 (kg/m2) is the most widely used criterion for obesity [1]. A BMI above 30 kg/m2 and above 35 kg/m2 with co-morbidities or above 40 kg/m2 are diagnostic criteria of obesity and morbid obesity, respectively. World-wide data shows that there is a continuous surge of obesity with 2/3 of USA adults obese and overweight (BMI>25 kg/m2) [2]. Israel is following the same international trend [3]. The prevalence of morbid obesity is reaching up to 10% of certain populations with women, minorities and low income sectors affected mostly by this disorder [4].Obesity raises a great concern since it is associated with accelerated morbidity and mortality which are preventable. The leading consequences of obesity are cardiovascular diseases and T2DM. Hypertension, pulmonary dysfunction, hyperlipidemia with low HDL-C are all contributing to cardiovascular morbidity. While as many as 90% of patients with T2DM are obese, lifestyle interventions and medications have not succeeded to confer a consistent protection against cardiovascular events in these patients [5].
Bariatric surgery is employed for patients with severe obesity (BMI≥40 kg/m2) or those with a lesser degree of obesity with comorbidity (BMI≥35 kg/m2). At our medical center, two procedures are often used: sleeve gastrectomy which reduces the stomach to about 25% of its original size and gastric bypass procedure. Laparoscopic approach is mostly undertaken [6].
In opposite to conventional non-surgical modest weight loss, bariatric surgery results in inhibition of appetite despite low caloric intake, changes in food preferences and major alternations in the hormones associated with food and glucose homeostasis. These alterations are apparently leading to a better endocrine pancreatic function and improved food control. These mechanisms may explain weight loss maintenance and consequent reduction in long term morbidity which is far beyond the negative caloric balance achieved by surgery [7-10].
In this study we have attempted to follow patients undergoing bariatric surgery for morbid obesity with and without diabetes. Since the evolution of atherosclerosis involves slowly operating mechanisms, we have assessed both metabolic and inflammatory parameters. It is well accepted that early atherosclerosis may be manifested by enhanced carotid arteries wall thickness, we have measured it before and after surgery [11,12].
Materials and Methods
Patients and study designOver 12 month’s period, 22 consecutive patients were studied prospectively. All these patients were evaluated by multi-disciplinary team and all met the accepted criteria for surgical intervention: a BMI≥40 kg/m2 or ≥35 kg/m2 with at least one obesity related comorbidity and failure of previous attempts to lose weight. Excess Weight Loss (EWL) was calculated by the following equation: BMI post surgery minus 25 (Ideal BMI) divided by pre-surgery BMI (%). Patients were divided into two groups: 11 with T2DM according to ADA criteria [13,14] and 11 without diabetes. 14 patients underwent sleeve gastrectomy and 8 underwent gastric bypass. The study was approved by the Ethics Committee of Rambam Health Care Campus according to Helsinki declaration and a written informed consent was obtained from each patient.
Determination of blood tests
Total cholesterol, triglycerides (TG), HDL-C, creatinine, urea, liver function tests and glucose were analyzed on HITACHI 7020 Chemistry analyzer. LDL-C was calculated by the Friedwald formula [15]. CRP was analyzed by a latex-enhanced-immuno nephelometry on Behring BNI Nephelometer [16]. IL-6 and TNF-α were determined by ELISA immunochemistry. All these tests were performed in the clinical biochemistry department which adheres to strict quality control standards. Complete blood count was performed with standard techniques. HbA1C was analyzed by a chromatography method with a D-10 analyzer.
Carotid intima-media thickness (IMT) ultrasound examination
The examination was done with the head hyper extended slightly and turned to the opposite side. Both axial and sagital scans were obtained in order to identify wall thickness and presence of plaques in the carotid arteries. IMT was calculated after imaging of the far wall of the carotid arteries which produced two echogenic lines: the lumen-intima interface and the media-adventitia interface. IMT is constituted by the combined thickness of the intima and media layers of the carotid walls measurements and performed on the distal 1cm of common carotid artery on each side. Electronic calipers were manually located on the two echogenic lines. Three measurements were taken from each sagital scan and the obtained values averaged. Mean values for each side were reported and when plaques were present their location and size were reported.
Ultrasound equipment used was a Philips HD 5000 and Philips IU 22 with a high resolution 5-17MHz linear array transducer. All measurements were performed by skilled personnel in the US unit according to an international consensus statement [17].
Data analysisThe normally of the data was tested by Kolmogorov Smirnov test. As some of the quantitative variables were not normally distributed, the non parametric tests were used. Wilcoxon signed ranks test was used for differences between baseline vs. three month follow-up in the quantitative variables. Repeated measure analysis was conducted to determine whether there was a statistical significant between two different types of surgery for changes in different quantitative variables. McNemar-Bowker Test was used for differences between baselines vs. three month in the categorical variables. Mean differences between baselines vs. three month was calculated for all the quantitative variables. Mann Whitney U tests were used to study ifferences between two in depended groups. P (SPSS, Chicago, IL).
Results
Weight responseTwenty two patients, 10 men and 12 women, mean age 41±13 years, range 20-64 years completed the study. Mean hospital stay was 4 days without notable adverse effects except patients who required blood transfusions for symptomatic blood loss and had prolonged hospital stay of 7 days. Mean body weight decreased from 126±24 to 102±21 kg and BMI decreased from 44.8±6.7 to 36.3±5.7 kg/m2, three month after surgery (p<0.0001). Overall, there was a 45.6±13.3% EWL. EWL was not influenced by gender, surgical procedure or diagnosis of T2DM.
General blood tests
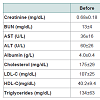
Table 1 summarized the effects of bariatric surgery on blood lipids, renal and liver function tests. According to Table 1, whereas creatinine and blood lipids were not altered by surgery, three month weight loss resulted in a significant drop in liver transaminases. Also, blood triglycerides dropped. A tendency towards mild elevation in blood BUN without influence on creatinine was noticed, which may have resulted from some changes in hydration status of patients after surgery.Inflammatory response
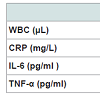
Table 2 illustrates the effects of surgery- induced-weight- loss on inflammatory markers including WBC and blood cytokines. Considering CBC, hemoglobin and platelets were not changed (Hb 13.7±1.3 and 13.6±1.2 g/l and platelets 247±69 and 245±61x103 before and after surgery, respectively). WBC decreased significantly, same about CRP. Other blood cytokines, IL-6 and TNF-α did not change throughout the study.Glycemic control
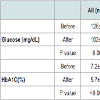
Table 3 demonstrates the effects of surgery on glucose homeostasis in the entire group, in the non-diabetic and in the diabetic groups of patients (11 in each group). Overall, there was a significant decrease in fasting blood glucose and HbA1C. However, in patients with T2DM, fasting glucose and HbA1C moved from a diabetic to a non-diabetic range with mean fasting blood glucose less than 126 mg/dL and mean HbA1C less than 6.5%. There were no significant differences between weight, BMI and extent of weight loss in patients with and without T2DM. Patients with T2DM had higher liver transaminases and triglycerides compared with non-diabetics. Other blood lipids were not different between the groups. Carotid arteries morphology
Carotid arteries examinations by ultrasound disclosed an IMT of 0.632±0.109 mm on the right side and 0.656±0.118 mm on the left side (mm). Three months after surgery, there was some increase in right sided IMT to 0.705±0.130 mm (p=0.008) without any change in the left side (0.669±0.118 mm, p=0.53). Before surgery, 5 patients were diagnosed with plaques. In one patient, plaque disappeared on the 3 month examination. Diagnosis of T2DM or surgical procedure had no effects on IMT results, before and after surgery.
Discussion
Our study demonstrates the early effects of bariatric surgery for treatment of morbid obesity. At a time interval of 3 month, approximately 50% EWL was followed by a significant improvement in liver enzymes, fasting glucose, HbA1C and inflammatory markers, still without such changes in carotid arteries morphology. Our patients underwent one of two common procedures: sleeve gastrectomy, which is a mainly restrictive procedure with some established metabolic effects and gastric bypass which has combined restrictive, mal-absorbtive and metabolic effects. Both procedures resulted in an impressive weight loss already documented after 3 months period without notable side effects. Weight loss surgery results in an improvement in blood lipids and dyslipidemia, with most of the patients able to discontinue hypo-lipidemic drugs postsurgery [18]. Similar to previously reported results, triglycerides were most responsive to surgery in our patients. HDL-C did not change in our patients, which is probably explained by the short-term follow up in and perhaps, its better response to restrictive procedures [19]. As mentioned in previous series, total and LDL-C were not affected by surgery in our patients [20].Obesity is responsible to non-alcoholic fatty liver disease characterized by the accumulation of triglycerides, active inflammation and progression into fibrosis, liver failure and even malignancy. Bariatric surgery with its accompanying beneficial metabolic effects and changes in hormonal and cytokines milieu has proven to be a very effective therapy, preventing progression of liver pathology. The significant reduction in liver transaminases in our patients as early as 3 month after surgery is in accordance with previous reports [21,22].
Obesity is associated with a pro-inflammatory state resulting from the production of diverse molecules by the adipose organ either directly or through liver synthesis. We have repeatedly demonstrated that obesity was the major determinant of elevated CRP in subjects with the metabolic syndrome, elevation of liver enzymes was associated with higher CRP, same about the association between fasting glucose and CRP [16,23,24]. Previous studies have showed a consistent reduction of more than 50% in CRP levels after bariatric surgery. CRP decreased relatively to the amount of weight loss achieved. However, IL-6 levels did not behave same direction in all studies and TNF-α was not influenced by surgery [25,26]. CRP decreased significantly in our patients without any changes in IL-6 and TNF-α. WBC decreased significantly in our study, confirming the usefulness of WBC count as a sensitive biomarker of inflammation [27].
Bariatric surgery appears to be the most effective intervention remedy in preventing and curing T2DM [28,29]. Bradely et al. investigated the response to a restrictive (banding) vs malabsorptive (gastric bypass) procedures [30]. Despite marked differences in hormonal response, both procedures resulted in similar decrease in markers of inflammation and improvement in glucose homeostasis. In other studies, sleeve gastrectomy resulted in T2DM resolution in up to 96% of patients with a drop of HbA1C from 8.4 to 6.1%. Our patients with T2DM showed almost the same early response to surgery: notably is the 46% change in EWL that was associated with the achievement of non-diabetic fasting glucose, (HbA1C shifted from 8.2 to 5.9%) in three month period. Also, there was a significant improvement in glucose homeostasis in the entire group and in the non-diabetic group. These results were not dependent upon age, gender or surgical procedure employed.
Risk assessment of atherosclerosis is based on tables like the Framingham score. The prediction of cardiovascular morbidity by non-invasive techniques such as CT or MRI has developed over the last years. Carotid IMT is an accepted and standartisized approach for more than 20 years being an excellent preclinical tool with good correlations with future coronary and cerebral events [31]. Moreover, IMT is considered a practical non-invasive modality in nutritional and pharmacological interventional studies. Supplementation with pomegranate juice or n-3 fatty acids resulted in favorable effects on IMT [32,33]. Recent studies showed an increased IMT in extreme obesity with a promising response in carotid wall indices in response to mild weight reduction [34-36]. Three month after surgery, no beneficial changes in IMT were observed in our patients except in one patient in whom plaque disappeared.
Limitations of study: our study followed a small number of patients and was conducted for a short period of time. Thus, it may not represent the long term maximal response to surgery. Also, sustainability of weight loss is required for long term improvement. However, in spite of the short time interval and the small number of patients, a prominent metabolic, inflammatory and glycemic response to surgery was evident.
Our results give strong support to the current evidence on the potential cure of T2DM by bariatric surgery [37]. Changes in liver enzymes, triglycerides and markers of inflammation, all may explain better survival in patients with extreme obesity after surgery [38,39]. Such treatment achievements may support implementation of lower body mass threshold criteria to surgery in patients with T2DM [40].
Acknowledgements
We would like to thank Ronit Leiba MSc for data analysis, Ortal Bar-On MSc for research coordination and Iris Coen for manuscript preparation.References
- Ahima RS, Lazar MA (2013) Physiology. The health risk of obesity – better metrics imperative. Science 341: 856-858.
- Hatori A, Strum R (2013) The obesity epidemic and changes in self-report biases in BMI. Obesity (Silver Spring) 21: 856-860.
- Kaluski DN, Berry EM (2005) Prevalence of obesity in Israel. Obes Rev 6: 115-116.
- Hensrud DD, Klein S (2006) Extreme obesity: a new medical crisis in the United States. Mayo Clin Proc 81: S5-S10.
- Sharma AM (2008) The value of current interventions for obesity. Nat Clin Pract Cardiovasc Med Suppl 1: S3-S9.
- Sakran N, Goiten D, Raziel A, Keidar A, Beglaibter N, et al. (2013) Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc 27: 240-245.
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, et al. (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724-1737.
- Shah M, Shimha V, Garg A (2006) Review: long-term impact of bariatric surgery on body weight, comorbidies, and nutritional status. J Clin Endocrinol Metab 91: 4223-4231.
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, et al. (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741-752.
- Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, et al. (2012) Bariatric surgery and long-term cardiovascular events. JAMA 307: 56-65.
- Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, et al. (2010) An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb 17: 1-11.
- Nguyen-Thanh HT, Benzaquen BS (2009) Screening for subclinical coronary artery disease measuring carotid intima media thickness. Am J Cardiol 104: 1383-1388.
- Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, et al. (2010) Screening for diabetes and pro-diabetes with proposed A1C based diagnostic criteria. Diabetes Care 33: 2184-2189.
- American Diabetes Association (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 27 suppl 1: S64-S71.
- Friedewald WT, Levy RL, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499-502.
- Aronson D, Bartha P, Zinder O, Kerner A, Markewitcz W, et al. (2004) Obesity is the major determinant of elevated c-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord 28: 674-679.
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, et al. (2008) Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media thickness task force. Endorsed by the Society for vascular medicine. J Am Soc Echocardiogr 21: 93-111.
- Nguyen NT, Varela E, Sabio A, Tran CL, Stamos M, et al. (2006) Resolution of hyperlipidemia after laparoscopic Roux-en-Y gastric bypass. J Am Coll Surg 203: 24-29.
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, et al. (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724-1737.
- Dixon JB, O'Brien PE (2002) Lipid profile in the severely obese: changes with weight loss after lap-band surgery. Obes Res 10: 903-910.
- Birza MA, Romeo S, Kotronen A, Svensson PA, Sjoholm K, et al. (2013) Long-term effect of bariatric surgery on liver enzymes in the Swedish Obese Subjects (SOS) study. PloS One 8: e60495.
- Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, et al. (2006) Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology 130: 1564-1572.
- Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, et al. (2005) Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Bio 25: 193-197.
- Aronson D, Bartha P, Zinder O, Kerner A, Shitman E, et al. (2004) Association between fasting glucose and C-reactive protein in middle-aged subjects. Diabet Med 21: 39-44.
- Agrawa V, Krause KR, Chengelis DL, Zalesin KC, Rocher LL, et al. (2009) Relation between degree of weight loss after bariatric surgery and reduction in albuminuria and C-reactive protein. Sur Obes Relat Dis 5: 20-26.
- Rao SR (2012) Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res 61: 789-807.
- Rogowski O, Shpaira I, Berliner S (2008) Exploring the usefulness of inflammation-sensitive biomarkers to reveal potential sex differences in relation to low-grade inflammation in individuals with the metabolic syndrome. Metabolism 57: 1221-1226.
- Gill RS, Karmali S, Sharma AM (2011) Treating type 2 diabetes mellitus with sleeve gastrectomy in obese patients. Obesity (Silver Spring) 19: 701-702.
- Dixon JB, Le Rous CW, Rubino F, Zimmet P (2012) Bariatric surgery for type 2 diabetes. Lancet 379: 2300-2311.
- Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, et al. (2012) Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 122: 4667-4674.
- Simon A, Megnien JL, Chrioni G (2010) The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol 30: 182-185.
- Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, et al. (2004) Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr 23: 423-433.
- He K, Liu K, Daviglus ML, Mayer-Davis E, Jenny NS, et al. (2008) Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am J Clin Nutr 88: 1111-1118.
- Freedman DS, Patel DA, Srinvasan SR, Chen W, Tang R, et al. (2008) The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 32: 749-756.
- Moore XL, Michell D, Lee S, Skilton MR, Nair R (2013) Increased carotid intima-media thickness and reduced distensibility in human class III obesity: independent and differential influences of adiposity and blood pressure on the vasculature. PloS One 8: e53972.
- Shai I, Spence JD, Schwarzfuchs D, Henkin Y, Parraga G, et al. (2010) Dietary intervention to reverse carotid atherosclerosis. Circulation 121: 1200-1208.
- Varela JE (2011) Bariatric surgery: a cure for diabetes? Curr Opin Clin Nutr Metab Care 14: 396-401.
- Poirier P, Chair F, Cornier MA, Mazzone T, Faha D, et al. (2011) AHA scientific statement. Bariatric surgery and cardiovascular risk factors. A scientific statement from the American Heart Association. Circulation 123: 1683-1701.
- Vest AR, Heneghan HM, Schuer PR, Young JB (2013) Surgical management of obesity and the relationship to cardiovascular disease. Circulation 127: 945-959.
- Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AR, et al. (2013) Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA 309: 2250-2261.