Journal of Neurology and Psychology
Research Article
Verbal and Visuospatial Abilities in Alzheimer’s Patients
Lucia Serenella De Federicis*, Dina Di Giacomo, Manuela Pistelli and Domenico Passafiume
- Department of Clinical Medicine, Public Health, Life and Environmental Science, University of L’Aquila, Italy
*Address for Correspondence: De Federicisa LS, Department of Clinical Medicine, Public Health, Life and Environmental Science, University of L’Aquila, Italy, Tel: (+39) 388.6492951; E-mail: lsdefedericis@hotmail.it
Citation: LS De Federicis, D Di Giacomo, M Pistelli, D Passafiume. Verbal and Visuospatial Abilities in Alzheimer’s Patients. J Neurol Psychol. 2016; S(3):9.
Copyright © 2016 De Federicisa LS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Special Issue: 3
Submission: 13 November 2016 | Accepted: 19 December 2016 | Published: 23 December 2016
Abstract
Introduction: In normal ageing, cognitive performance changes according to the neuronal modifications that occur in the brain. The elderly, as compared to younger individuals, have better verbal than visuospatial performance. In Alzheimer’s Disease (AD), neuropathological changes generally produce disorders of memory, language and semantic knowledge. However, recent studies have shown that early-stage AD may present visuospatial deficits. The aim of this research is to study the cognitive changes of verbal and visuospatial performance that occur during AD.Keywords:
Alzheimer’s disease; Ageing; Cognitive Performances; Verbal abilities; Visuospatial abilitiesIntroduction
The concept of differential rates of maturation and decline for various cognitive functions has long been demonstrated. In recent years, researchers have hypothesized that functional reorganization in the brain is correlated with age [1]. Several studies have investigated the cognitive performances in healthy ageing [2,3], showing a more rapid loss of visual spatial than verbal skills [4], associate with a change on visual working memory [5], and a decline of divergent thinking [6]. The results of these studies suggest that functional reorganization of the brain occurs throughout the ageing process. Documented functional and structural neocortical hemispheric asymmetries in individuals with normal cognition [7-9], and a more rapid decline of the right than the left hemisphere corroborate this [10].Methods and Materials
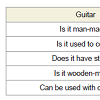
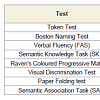
SubjectsTo evaluate the ability with visuospatial access were administered the following tests:
Results
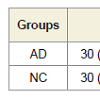
The NC group was statistically younger (p < 0.05) than the AD sample, although the age ranges and frequency distributions were similar, and the actual differences were quite small. There was no statistically significant difference between the levels of education of the two groups (p = 0.148). The gender distribution was studied by frequency table, and the distribution was not statistically significant within the two groups (p = 0.287). Subjects with AD performed significantly worse than the controls on all tests (p = 0.000, Mann-Whitney U Test) (Figure 2).Bonferroni’s post-hoc analysis revealed that the AD group’s VERB and SPAZ composite scores were both lower than the NC composite indexes. In both groups (AD and NC) there was a significant difference between the verbal and visuospatial indexes (p = 0.000), with the lowest spatial index than the verbal. Figure 3 shows the cognitive-performance trends of the two groups (Figure 3).
The ROC curve analysis was used to evaluate the accuracy of the new variables (VERB and SPAZ) to discriminate normal from pathological cases. For the VERB coefficient, the results showed high accuracy: AUC = 0.996, with 100% specificity and 93.1% sensitivity; similar results were obtained for the SPAZ coefficient: AUC = 1.000, with 100% specificity and 100% sensitivity, confirming the high accuracy of two variables to discriminate normal from pathological subjects. The Figure 4 shows the ROC curve of VERB and SPAZ coefficients (Figure 4).
To determine whether the interaction between groups and verbal and visuospatial abilities was due to the confounding influence of age, we conducted an analysis of covariance with age as the covariate. Analysis showed an interaction between groups and composite scores, F(1) = 65.30, p < 0.000, partial η2 = 0.542.
To determine whether the interaction between groups and verbal and visuospatial performances was due to the confounding influence of education, we conducted an analysis of covariance with the level of education as a covariate. The analysis again showed a significant interaction effect between groups and composite scores, F(1) = 77.52, p < 0.000, partial η2 = 0.584.
To evaluate the difference in verbal and visuospatial performance in mild and moderate dementia, we divided the pathological group on the basis of value of MMSE: mild deterioration, between 21-26; moderate, 14 to 20. The result is a group of n. 13 subjects with mild deterioration [age X = 75.15 (sd 6.17), education X = 6.61 (sd 3.37), MMSE X = 23.35 (sd 1.44)], and a group of n. 16 subjects with moderate deterioration [age X = 76.93 (sd 7.32), education X = 7.06 (sd 2.56), MMSE X = 18.02 (sd 1.92)]. The 2 x 2 ANOVA (mild and moderate groups x VERB and SPAZ composite scores) was conducted to compare the performances of two pathological groups. The analysis demonstrated a main effect for the groups, F(1) = 9.59, p = 0.004, partial η2 = .26, and of the composite scores, F(1) = 3157.36, p = 0.000, partial η2 = 0.99, but not a significant interaction between the groups and the composite scores, F(1) = 0.101, p = 0.752, partial η2 = 0.003, confirming the presence of a severe visuospatial deficits in the mild stage of disease.
Discussion
The aim of this work was to model the cognitive decline in early stage of Alzheimer’s Disease, verifying the difference between verbal and visuospatial abilities in AD subjects. Two samples of subjects one composed of subjects with mild-to-moderate AD and one of NC subjects free from neurological pathology-were compared on MMSE, standardized cognitive screening test, and two experimental tasks. Neuropsychological tests have been divided according to ability and/or the brain areas involved, balancing them to access verbal and visuospatial, and, for not to influence the performance of the subjects, we excluded tests of short and long term memory, except tasks of semantic memory.In line with our hypothesis, the findings indicate that AD, compared to controls, affects both verbal than visuospatial abilities in early stage of disease. Moreover, the cognitive impairment does not to follow a homogeneous trend: the visuospatial abilities seem to suffer a more rapid deterioration. The findings suggested that visuospatial input was more efficient than verbal input to detect patients who were at the early stage of cognitive decline. This finding is consistent with the studies of Johnson [35], which suggested that visuospatial deficit may occur early, even in preclinical stages. However, a large number of studies have found verbal performance deficits in the early stages of dementia [27]. An explanation for this trend could be derived from the analysis of the studies considered in a review by Collie & Maruff [28]. The authors highlight the widespread use in the early diagnosis of tests that involve verbal abilities. Our analysis, however, showed high accuracy to discriminate normal from pathological subjects both verbal tasks than visuospatial ones. Greater detection of verbal deficit in the early stages of disease may indeed depend on large use, in clinical practice, the verbal tasks that usually require most and simple administration. Furthermore, since not supported by the language and verbal reasoning, visuospatial tasks may be more difficult to resolve.
A growing body of research suggests that subtle cognitive changes during the clinical and preclinical phase of AD can be detected as brain asymmetry on cognitive tasks [61]. Jacobson and collaborators, in accordance with this theory, studies 20 cognitively normal elderly adults who were in a preclinical phase of AD and compared them to 20 age- and education-matched normal control subjects through a series of cognitive tests. They found a statistically difference between verbal (Boston Naming Test) and spatial (Test Block Design) standardized scores, supporting the thesis of cognitive asymmetry and highlighting the utility of asymmetric cognitive profiles in identifying individuals at risk for AD [62]. On the other hand, some studies have shown that there is not always a correspondence between brain asymmetry in AD and cognitive performance. A recent study by Balasubramanian and collaborators [63], conducted on subjects with AD neuropathology and on the very elderly (over 90 years old) with no dementia, reported that AD neuropathology at autopsy was not associated with the trajectory of cognitive performance. The authors found no significant difference in cognitive performance over time, based on plaque or tangle staging, and suggested searching for causes other than AD neuropathology that may affect cognition in the very elderly. However, several researches documented a reduction of brain asymmetry in AD subjects [64]. Studies about the lateralization of cognitive deficits in subgroups of AD patients with mild dementia showed that discrepancies between language and visuospatial deficits in patients with early AD are related to asymmetrical reductions in cerebral cortical [65], especially in the inferior parietal lobule [66]. Similar results were obtained by studies on healthy elderly show a reduction of cerebral asymmetry [1,67]. By comparing regional brain activation of young adults and elderly subjects during tasks of episodic and semantic memory, it was shown that the young adults had activation of the left prefrontal cortex, while the elderly individuals experienced bilateral activation of prefrontal cortex [1]. Similar results were obtained by Stebbins [67], who demonstratedthat young people, on both abstract and concrete-language tasks, had almost double the activation in the left prefrontal cortex than in the right, in comparison, the asymmetry of activation disappeared among the elderly participants. In healthy seniors, therefore, there is a reduction of cerebral asymmetry, meaning that, during cognitive tasks, there is activation in both the left and right hemispheres. Based on the above, in the current state of the art, it is not possible for us to declare with certainty that the early impairment of verbal and/or visuospatial abilities depend on the reduction of brain asymmetry, this thesis needs more confirmations.
Another aspect that should be further investigated is the role of the APOE genotype in the evolution of brain atrophy [68,69]. Some authors claim that the heterogeneity of symptoms could be explained by apolipoprotein E (APOE): the 3 APOE genotype modifies the clinical phenotype in terms of cognitive impairment and is predictive of progression to disease [70,71]. Wolk & Dickerson studied APOE carriers and non-carriers and report a strong relationship between performances in specific cognitive domains and neuroanatomical changes in the regions that support those functions, while Donix and his collaborators have shown that the APOE-4 allele modulates hemispheric asymmetry in entorhinal cortical thickness [72,73]. Additionally, Hashimoto and colleagues reported different patterns of regional brain atrophy among patients with different APOE genotypes, meaning that the effect of APOE epsilon 4 gene may have regionally specific effects on the brains of AD patients [74]. The results of these studies are very encouraging and we believe that they represent one of the ways to follow for clarifying the nature and extent of cognitive deficits in AD.
In a study of 2011 about verbal and visuospatial performances in healthy subjects, the authors showed that with increasing age there was a different use of cognitive skills, characterized by early development of visuospatial skills compared to a slower specialization of verbal abilities, which reach full maturity in adulthood. This trend is also preserved in old age, albeit with less accurate performance than are possible in adulthood [4]. Even though it is difficult to make a strong conclusion based on the small number of AD patients studied in the current investigation, our findings indicate that the trends of cognitive impairment in AD follow cognitive trends seen in healthy aging subjects. Based on this assumption, and the data we obtained, it could be argued that the ability to mature and specialize before, it is also the one that deteriorates earlier. This is in accordance with some researches that showed a more rapid greater age-related decline of the right than the left hemisphere [10]. Verify this hypothesis means to clarify one of the fundamental questions of neuroscience: how the architecture of the brain supports the complexities of cognitive functions. Several studies using modern neuroimaging techniques and neurocognitive test batteries to observe simultaneously changes in neuroanatomy and cognitive function as children mature into adults. Among them, we find a study of 2013 by Denninson and collaborators. The authors have shown that the left hemisphere was consistently larger than the right in subjects 12 to 16 years of age, and their results suggest that subcortical brain development from early to middle adolescence is characterized by striking hemispheric specialization [75]. Further researches are needed to identify the role of maturation and specialization of cerebral hemispheres in cognitive performance in lifespan.
Numerous studies have found a connection between age, education, gender, and cognitive performance in both healthy subjects and those with AD [3,76-78]. However, our analysis does not suggest this correlation, though this result could have been affected by a small sample size.
Finally, we did not take into consideration the role of cognitive reserve. In the literature there are several studies which have shown a correlation between level of cognitive deterioration and cognitive reserve and the role of cognitive reserve in the evolution of dementia [79,80]. Further development of this research could help clarify if levels of cognitive reserve differentially influence the execution of verbal and visuospatial tasks.
In conclusion, our results suggest that, in AD, both the verbal than visuospatial skills deteriorate in early stage of disease, and visuospatial abilities seem to have a more rapid impairment. The findings suggest that visuospatial access was more efficient than verbal access to detect patients who were at the early stage of cognitive decline. These results are not in line with a large part of research in the field which considers the verbal deficits as early signs of dementia. This difference in results may be due to a large use of test with verbal access in clinical practice, which is of faster and easier administration. It is still necessary to deepen the role of maturation and specialization of the cerebral hemispheres and of modification of the brain asymmetry, which seems to reduce with age. Further research needs to identify more sensitive instruments to detection visuospatial abilities in the early phases of dementia. The confirm of the early impairment is crucial for early detection of the disease and opens the way to several interventions of prevention and treatment. The knowledge resulting from these researches could be used for the construction of more sensitive non-invasive diagnostic tests for the early detection of dementia, and for the implementation of informatics applications of cognitive stimulation intended for subjects with subtle cognitive changes.
Funding
This work was supported by a grant from MIUR, the Mesva Department (ex 60% of University of L’Aquila).Acknowledgements
We would like to thank Professor Stefano Necozione, from Dept Clinical Medicine, Public Health, Life and Environmental Science, University of L’Aquila, for his assistance in statistical analysis of data, and Dr. Micaela Andrich for the editing support.References
- Cabeza R (2002) Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17: 85-100.
- Ojeda N, Aretouli E, Peña J, Schretlen DJ (2014) Age differences in cognitive performance: a study of cultural differences in historical context. J Neuropsychol 10: 104-115.
- van Hooren SA, Valentijn AM, Bosma H, Ponds RW, van Boxtel MP, et al (2007) Cognitive functioning in healthy older adults aged 64-81: a Cohort study into the effects of age, sex, and education. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14: 40-54.
- De Federicis LS, Pistelli M, Fiorenzi D, Di Giacomo D, Passafiume D (2011) Differenze tra abilità linguistiche e visuo-spaziali nell'invecchiamento. Psicogeriatria 2: 27-37.
- Brockmole JR, Logie RH (2013) Age-related change in visual working memory: a study of 55,753 participants aged 8-75. Front Psychol 4: 12.
- Palmiero M (2015) The effects of age on divergent thinking and creative objects production: a cross-sectional study. High Abilitiy Study 26: 93-104.
- Allen JS, Bruss J, Mehta S, Grabowski T, Brown CK, et al. (2008) Effects of spatial transformation on regional brain volume estimates. Neuroimage 42: 535-547.
- Gershwhin N, Galaburda AM (1985) Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol 42: 428-459.
- Toga AW, Thompson PM (2003) Mapping brain asymmetry. Nat Rev Neurosci 4: 37-48.
- Dolcos F, Rice HJ, Cabeza R (2002) Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev 26: 819-825.
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 3: 186-191.
- Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82: 239-259.
- Kim JH, Lee JW, Kim GH, Roh JH, Kim MJ, et al. (2012) Cortical asymmetries in normal, mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging 33: 1959-1966.
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, et al. (2001) Patterns of cognitive decline in presymptomatic Alzheimer's disease: a perspective community study. Arch Gen Psychiatry 58: 853-858.
- Perry RJ, Watson P, Hodges JR (2000) The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer's disease: relationship to episodic and semantic memory impairment. Neuropsycologia 38: 252-271.
- Chang YL, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ Jr, et al. (2010) Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer's disease. Neuropsychologia, 48: 1237-1247.
- Genon S, Collette F, Moulin CJ, Lekeu F, Bahri MA, et al (2013) Verbal learning in Alzheimer's disease and mild cognitive impairment: fine-grained acquisition and short-delay consolidation performance and neural correlates. Neurobiol Aging 34: 361-373.
- Carlesimo GA, Oscar-Berman,M (1992) Memory deficits in Alzheimer's patients: a comprehensive review. Neuropsychol Rev 3: 119-169.
- Salmon DP, Bondi MW (1999) Neuropsychology of Alzheimer's disease. In Alzheimer Disease, Terry RD, Katzman R, Bick KL, Sisodia SS, Eds., Lippincott Williams & Wilkins, Philadelphia, USA. pp. 39-56.
- Belleville S, Chertkow H, Gauthier S (2007) Working memory and control of attention in person with Alzheimer's disease and mild cognitive impairment. Neuropsychology 21: 458-469.
- Duke LM, Kasniak AW (2000) Executive control functions in degenerative dementias: a comparative review. Neuropsychol Rev 10: 75-99.
- Cuetos F, Arango-Lasprilla JC, Uribe C, Valencia C, Lopera F (2007) Linguistic changes in verbal expression: a preclinical marker of Alzheimer’s disease. J Int Neuropsychol Soc 13: 433-439.
- Szatloczki G, Hoffmann I, Vincze V, Kalman J, Pakaski M (2015) Speaking in Alzheimer's disease, is that an early sign? Importance of change in language abilities in Alzheimer's disease. Front Aging Neurosci 7: 195.
- Chertkow H, Bub D, Schwartz M (1990) Semantic memory loss in Alzheimer type dementia. In Modular deficits in Alzheimer-type dementia, Schwartz MF, Ed., MIT Press, Cambridge, UK. pp. 207-244.
- Di Giacomo D, De Federicis LS, Pistelli M, Fiorenzi D, Sodani E, et al. (2012) The loss of conceptual associations in mild Alzheimer's dementia. J Clin Exp Neuropsychol 34: 643-53.
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, et al. (2005) Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol 62: 1601-1608.
- Arnaiz E, Almkvist O (2003) Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Acta Neurol Scand Suppl 179: 34-41.
- Collie A, Maruff P (2000) The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neurosci Biobehav Rev 24: 365-374.
- Bublak P, Redel P, Sorg C, Kurz A, Forstl H, et al. (2011) Staged decline of visual processing capacity in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 32: 1219-1230.
- Parks RW, Thiyagesh SN, Farrow TF, Ingram L, Wilkinson K, et al. (2010) Performance on the clock drawing task correlates with FMRI response to a visuospatial task in Alzheimer’s disease. Int J Neurosci 120: 335-343.
- Locascio JJ, Growdon JH, Corkins S (1995) Cognitive test performance in detecting, staging, and tracking Alzheimer's disease. Arch Neurol 52: 1087-1099.
- Festa EK, Insler RZ, Salmon DP, Paxton J, Hamilton JM, et al. (2005) Neocortical disconnectivity disrupts sensory integration in Alzheimer's disease. Neuropsychology 19: 728-738.
- Paxton JL, Peavy GM, Jenkins C, Rice VA, Heindel WC, et al. (2007) Deterioration of visual-perceptual organization ability in Alzheimer's disease. Cortex 43: 967-975.
- Yassa MA, Verduzco G, Cristinzio C, Bassett SS (2008) Altered fMRI activation during mental rotation in those at genetic risk for Alzheimer's disease. Neurology 70: 1898-1904.
- Johnson DK, Storandt M, Morris JC, Galvin JE (2009) Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol 66: 1254-1259.
- Bäckman L, Jones S, Berger AK, Jonsson Laukka E, Small BJ (2005) Cognitive impairment in preclinical Alzheimer's d isease: a meta-analysis. Neuropsychology 19: 520-531.
- Hodges JR (2006) Alzheimer’s centennial legacy: origins, landmarks and the current status of knowledge concerning cognitive aspects. Brain 129: 2811-2822.
- Godbolt AK, Cipolotti L, Watt H, Fox NC, Janssen JC, et al. (2004) The natural history of Alzheimer's disease: a longitudinal presymptomatic and symptomatic study of a familial cohort. Arch Neurol 61: 1743-1748.
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, et al. (2011) Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 280-292.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work group under the auspice of Department of Health and Human Service Task Force on Alzheimer’s disease. Neurology 34: 939-944.
- Folstein MF, Folstein SE, McHugh PR (1995) Mini-mental state. J Psychol Res 12: 189-198.
- De Renzi E, Vignolo LA (1962) The token test: a sensitive test to detect receptive disturbances in aphasia. Brain 85: 665-678.
- Spinnler H, Tognoni G (1987) Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci 8.
- Mendez MF, Clark DG (2008) Neuropsychiatric aspects of aphasia and related disorders. In Textbook of neuropsychiatry and behavioural neurosciences, Yudofsky SC, Hales RE, Eds., American Psychiatric Publishing, Arlington, USA.
- Kaplan EF, Goodglass H, Weintraub S (1983) The Boston Naming test (2nd edn). Lea & Febiger, Philadelphia, USA.
- Lezak MD, Howieson DB, Bigler ED, Tranel D (2012) Neuropsychological Assessment. Fifth Edition. Oxford University Press, USA.
- McDowd J, Hoffman L, Rozek E, Lyons KE, Pahwa R (2011) Understanding verbal fluency in healthy aging, Alzheimer's disease, and Parkinson's disease. Neurpsychology 25: 210-225.
- Carlesimo GA, Calatagirone C, Gainotti G (1995) ed il gruppo per la strandardizzazione della batteria per il deterioramento mentale. In Batteria per la valutazione del deterioarmento mentale (parte II): standardizzazione ed affidabilità diagnostica nell'identificazione die pazienti affetti da sindrome demenziale. Arch Psicol Neurol Psichiatr 56: 489-502.
- Caltagirone C, Gainotti G, Carlesimo GA (1995) ed il gruppo per la strandardizzazione della batteria per il deterioramento mentale. In Batteria per la valutazione del deterioarmento mentale (parte I): descrizione di uno strumento di diagnosi neuropsicologica. Arch Psicol Neurol Psichiatr 56: 461-470.
- Giffard B, Desgranges B, Nore-Mary F, Lalevée C, de la Sayette V, et al. (2001) The nature of semantic memory deficits in Alzheimer’s disease: New insights from hyperpriming effects. Brain 124: 1522-1532.
- Raven JC (1995) Coloured Progressive Matrices Sets A, Ab, B. Manual sections 1 & 2. Oxford: Oxford Psychologists Press, UK.
- Weiner PS, Wepman JM, Morency A (1965) A test of visual discrimination. Elem Sch J 65: 330-337.
- Ekstrom RB, French JW, Harman HH, Dermen D (1976) Manual for kit of factor-referenced cognitive tests. Educational Testing Service, Princeton.
- Goodglass H (1980) Disorders of naming following brain injury. Am Sci 68: 647-655.
- De Federicis LS (2004) Deficit delle categorie semantiche nella demenza di Alzheimer. PhD dissertation
- Di Giacomo D, De Federicis LS, Pistelli M, Fiorenzi D, Passafiume D (2012) Semantic associative relations and conceptual processing. Cogn Process 13: 55-62.
- Joubert S, Brambati SM, Ansado J, Barbeau EJ, Felician O, et al. (2010) The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 48: 978-988.
- Ferguson CJ (2009) An effect size primer: a guide for clinicians and researchers. Prof Psychol Res Pr 40: 532-538.
- Swets JA (1998) Measuring the accuracy of diagnostic system. Science 240: 1283-1293.
- AAVV. Statistic, IInd Edition, Statsoft, 1998.
- Almkvist O (2000) Functional brain imaging as a looking-glass into the degraded brain: reviewing evidence from Alzheimer disease in relation to normal aging. Acta Psychol 105: 255-277.
- Jacobson MW, Delis DC, Bondi MW, Salmon DP (2002) Do neuropsychological tests detect preclinical Alzheimer’s disease? Individual-test versus cognitive discrepancy analyses. Neuropsychology 16: 132-139.
- Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM (2012) Alzheimer's disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology 79: 915-921.
- Long X, Zhang L, Liao W, Jiang C, Qiu B (2013) Distinct laterality alterations distinguish mild cognitive impairment and alzheimer's Disease from healthy aging: statistical parametric mapping with high resolution MRI. Hum Brain Mapp 34: 3400-3410.
- Haxby JV, Duara R, Grady CL, Cutler NR, Rapoport SI (1995) Relations between neuropsychological and cerebral metabolic asymmetries in early Alzheimer’s disease. J Cereb Blood Flow Metab 5: 193-200.
- Kim JH, Lee JW, Kim GH, Roh JH, Kim MJ, et al. (2012) Cortical asymmetries in normal, mild cognitive impairment, and Alzheimer's disease. Neurobiol Aging 33: 1959-1966.
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, et al. (2002) Aging effects on memory encoding in the frontal lobes. Psychol Aging 17: 44-55.
- Pievani M, Galluzzi S, Thompson PM, Rasser PE, Bonetti M, et al. (2011) APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer's disease. Neuroimage 55: 909-919.
- Geroldi C, Pihlajamäki M, Laakso MP, De Carli C, Beltramello A, et al. (1999) APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology 53: 1825-1832.
- van der Vlies AE, Pijnenburg YA, Koene T, Klein M, Kok A, et al. (2007) Cognitive impairment in Alzheimer's disease is modified by APOE genotype. Dement Geriatr Cogn Disord 24: 98-103.
- Morgen K, Frölich L, Tost H, Plichta MM, Kölsch H, et al. (2013) APOE-Dependent phenotype in subjects with mild cognitive impairment converting to Alzheimer's disease. J Alzheimers Dis 37: 389-401.
- Wolk DA, Dickerson BC, Alzheimer's Disease Neuroimaging initiative (2010) Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A 107: 10256-10261.
- Donix M, Burggre AC, Schar M, Maschner K, Suthana NA, et al. (2013) APOE associated hemispheric asymmetry of entorhinal cortical thickness in aging and Alzheimer's disease. Psychiatry Res 214: 212-220.
- Hashimoto M, Yasuda M, Tanimukai S, Matsui M, Hirono N, et al. (2001) Apolipoprotein E epsilon4 and the pattern of regional brain atrophy in Alzheimer's disease. Neurology 57: 1461-1466.
- Denninson M, Whittle S, Yücel M, Vijayakumar N, Kline A, et al. (2013) Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev Sci 16: 772-791.
- Lemaître H, Crivello F, Grassiot B, Alpérovitch A, Tzourio C, et al. (2005) Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage 26: 900-911.
- Moreno-Martínez FJ, Laws KJ, Schulz J (2008) The impact of dementia, age and sex on category fluency: greater deficits in women with Alzheimer's disease. Cortex 44: 1256-1264.
- Irvine K, Laws KR, Gale TM, Tejinder KK (2012) Greater cognitive deterioration in women than men with Alzheimer's disease: a meta analysis. J Clin Exp Neuropsychol 34: 989-998.
- Stern Y (2009) Cognitive reserve. Neuropsychologia 47: 2015-2028.
- Stern Y (2012) Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 11: 1006-1012.