Journal of Neurology and Psychology
Download PDF
Special Issue: Clinical Neuropsychology
Research Article
*Address for Correspondence: David A.S. Kaufman, Ph.D., Department of Psychology, Saint Louis University, Morrissey Hall 2739, 3700 Lindell Blvd, St. Louis, MO 63108, USA, E-mail: dkaufma3@slu.edu
Citation: Van Patten R, Kaufman DAS, Mitchell S, Sachs B, Loring DW. Perseverative Error Subtypes in Patients with Alzheimer’s Disease and Mild Cognitive Impairment. J Neurol Psychol. 2015; S(2): 9.
Copyright © 2015 Patten RV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Special Issue: 2
Submission: 7 October 2015 | Accepted: 27 November 2015 | Published: 03 December 2015
Editors:Dr. David AS Kaufman, Assistant Professor of Psychology, Saint Louis University, USA
Table 5 shows intercorrelations among the three GST indices, total perseverative errors, and select neuropsychological tests. Results indicate that, contrary to Lamar et al. [26], Semantic errors did not correlate significantly with measures of language functioning (the BNT and Animal Fluency), but did correlate significantly with several measures of EFs, including Digit Span Backward (r = -.38, p < .05), Trails B – A (r = .34, p < .05), COWA – FAS (r = -.49, p< .01), Luria Letter Alternation (r = .69, p< .001), and Clock Drawing Errors (r = .42, p< .01). As performance on these neuropsychological tests declined, Semantic perseverations tended to increase. Interestingly, and consistent with our hierarchical model of perseverations, Motor errors did not tend to correlate with these executive tasks, with the exception of Digit Span Backward (r = -.43, p< .01). Additionally, unlike Semantic or Motor errors, the Border error subtype correlated with LM I (r = -.39, p< .05) and LM II (r = -.33, p <.05), suggesting a sensitivity to memory decline across both MCI and AD patients.
Group by perseveration type
Research Article
Perseverative Error Subtypes in Patients with Alzheimer’s Disease and Mild Cognitive Impairment
Ryan Van Patten1, David A.S. Kaufman1*, Sandra Mitchell2, Bonnie Sachs3, and David W. Loring4,5
- 1Department of Psychology, Saint Louis University, St. Louis, MO, USA
- 2Alaska Neurology Center, Anchorage, AK, Canada
- 3Department of Neurology, Wake Forest University School of Medicine, Winston-Salem, NC, USA
- 4Department of Neurology, Emory University School of Medicine, Atlanta, GA, USA
- 5Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, USA
*Address for Correspondence: David A.S. Kaufman, Ph.D., Department of Psychology, Saint Louis University, Morrissey Hall 2739, 3700 Lindell Blvd, St. Louis, MO 63108, USA, E-mail: dkaufma3@slu.edu
Citation: Van Patten R, Kaufman DAS, Mitchell S, Sachs B, Loring DW. Perseverative Error Subtypes in Patients with Alzheimer’s Disease and Mild Cognitive Impairment. J Neurol Psychol. 2015; S(2): 9.
Copyright © 2015 Patten RV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Special Issue: 2
Submission: 7 October 2015 | Accepted: 27 November 2015 | Published: 03 December 2015
Editors:Dr. David AS Kaufman, Assistant Professor of Psychology, Saint Louis University, USA
Abstract
Introduction: Perseverations have been defined as repetitions of behaviors that are no longer congruent with task requirements. Perseverations often reflect global neuropathology and have been repeatedly elicited in individuals with neurodegenerative conditions such as Alzheimer’s disease (AD). We sought to demonstrate that subtypes of perseverations, as measured by the Graphical Sequence Test for Dementia (GST-D), would show differential, hierarchical relationships with tests of cognitive abilities and would discriminate between mild cognitive impairment (MCI) and AD.Methods: Participants were 16 patients with AD and 18 patients with MCI who completed neuropsychological evaluations in a memory disorders clinic. Assessment measures covered domains of verbal memory, language, executive functions (EFs), and frontal/motor symptoms. The GST-D was administered and perseverative errors were qualitatively scored and synthesized into three hierarchical constructs for analysis, with Semantic errors residing at the top of the hierarchy, followed by Border and Motor errors respectively.
Results: Collapsing across groups, data supported the hierarchical structure of GST-D errors such that Semantic errors tended to exhibit negative correlations with performance on measures of EFs, while Motor errors did not. Additionally, Border errors correlated negatively with verbal memory performance and showed a differential sensitivity to AD symptomatology when compared with Semantic and Motor errors.
Conclusions: Overall, results support theoretical conceptualizations of perseverative errors as existing both vertically, within a hierarchical framework, and horizontally, across neurocognitive domains. Notably, our evidence suggests that the production of Border errors may be driven by the progression of AD and, consequently, that measurement of perseverative error subtypes may have clinical utility in the assessment of individuals with AD.
Keywords:
Executive functions; Perseverative behavior; Graphical sequence test; Alzheimer’s disease; Mild cognitive impairmentIntroduction
Executive functions (EFs) are a heterogeneous family of topdown cognitive abilities that are frequently studied, yet often misunderstood [1]. Numerous tasks have been devised to measure executive aspects of behavior. One line of research has sought to elucidate the phenomenon of perseverative behavior (e.g., see [2]), which represents cognitive inflexibility and was originally defined as a repetition of a behavior that is no longer congruent with task requirements [3]. Perseverative behavior was originally discussed exclusively in the context of frontal lobe pathology (e.g., [4]). However, other evidence suggests that it can result from posterior lesions as well (e.g., [5]) and that it correlates positively with overall extent of brain damage and may reflect global rather than localized neuropathology [6]. A number of nosologies of perseverative behavior have been introduced to explain the various underlying facets reported in the literature (see [7] for a review). Moreover, these facets can be measured with a number of different instruments, including verbal fluency tasks [8], the Wisconsin Card Sorting Test [9], the California Verbal Learning Test [10] and other list learning tasks, as well as motor tasks designed to elicit perseverative behavior (e.g., [11]).The literature on perseverative behavior has expanded across time to include various neurological populations, including schizophrenia [12], addictive disorders (e.g., [13-15]) stroke and subsequent hemineglect [16-20], dementia of various etiologies [21-32], traumatic brain injury [33], and even healthy younger and older adults [34,35]. The reports of modest numbers of perseverative errors (i.e., perseverations that are determined to be incorrect responses) in these latter studies (i.e., [34,35]) are in line with the assertion by Allison that [36], while healthy individuals may produce some perseverations, notable or pronounced perseverative behavior is generally indicative of some form of neuropathology. Therefore, given that these errors are more frequent with greater neurological impairment, progressive neurodegenerative diseases that reduce overall brain volume and disrupt multiple domains of cognitive functioning logically lead to increased perseverations.
Alzheimer’s disease (AD) is the most common neurodegenerative disorder [37] and has been repeatedly associated with perseverative behavior. Indeed, Cabrera, Chavez, Corley, Kitto, and Butt posited a mechanism through which the neuropathology of AD might reduce mental flexibility using a rat model – namely [38], the degeneration of cholinergic neurons in the nucleus basalis of Meynert. Although AD is most frequently associated with memory impairments (e.g.,[39-43]), a growing body of literature suggests that attentional and executive control systems also break down early in the disease [44-48]. Extrapolating from these findings, it could be that such deficits underlie the perseverative behavior seen in AD. Moreover, as discussed by Hotz and Helm-Estabrooks [7], some researchers have postulated that memory impairments may serve as a mechanism for at least some types of perseverations. Therefore, taken together, the literature suggests that both memory and executive dysfunction may combine to underlie perseverative behavior in AD.
As reviewed above, several investigators have sought to operationalize subtypes of perseverations that are elicited by various neuropsychological instruments. Goldberg and Tucker introduced the Graphical Sequence Task (GST) [49], which has been used in several neurological populations and been shown to reliably elicit perseverations (e.g., [12,50]). Instruments such as the GST, specifically designed to elicit perseverations on visual-motor tasks, are valuable because 1) similar tasks have been shown to elicit more perseverations than standard neuropsychological tests in patients with brain injuries [33] and 2) less complex visual-motor tasks such as line cancellation have been shown to produce few perseverative responses in AD and may therefore be insensitive to the neurodegeneration in this disease [19].
Lamar and colleagues adapted the GST for individuals with dementia (the GST-D) and produced a hierarchical model [26], modified from the original Goldberg and Tucker nosology [49]. The authors labeled three subtypes of perseverations based on principal components analysis factor loadings: Semantic, Motor, and Border subtypes. Semantic perseverations represented dysregulation at the highest level of the cognitive hierarchy and included categorical incongruencies with respect to instruction (e.g., the examiner instructs the participant to write the word “triangle”) and participant output (e.g., the individual draws a triangle rather than writing the word “triangle”). In contrast, Motor perseverations represented lower-level errors such as fused geometric figures (e.g., the participant draws a hybrid circle-triangle figure following separate instructions to draw circles and triangles) or the continued production of graphic figures or letters despite explicit instructions from the examiner to discontinue the activity. Finally, Border perseverations included features of both semantic and motor subtypes and were conceptualized as representing the intermediate level of the hierarchy. Results from Lamar et al. demonstrated negative relationships between the semantic perseverations and performance on the Boston Naming Test and Animal Fluency in AD and between motor perseverations and the Finger-Tapping Test in vascular dementia [26], suggesting that these factors were indeed measuring high-level semantic and low-level motor skills, respectively. Moreover, the GST-D has been utilized to explicate the relationship between these three subtypes of perseverations and other measures of EFs [26], with these investigators concluding that Semantic and Border perseverations represent an inefficiency in working memory, a dimension of EFs [51], while Motor perseverations represent preparatory set.
The current study investigated the performance of individuals with AD, as well as Mild Cognitive Impairment (MCI; see [52]), on the GST-D and a battery of neuropsychological instruments designed to assess functioning in the domains of language, memory, EFs, and motor sequencing. MCI is conceptualized as a prodromal phase of dementia that is commonly associated with preclinical AD pathology [53]. MCI was originally defined as subjective and objective memory complaints, together with intact overall cognitive abilities, intact functional capacity, and the absence of dementia [54]. More recently, the construct has been broadened to include nonamnestic as well as amnestic subtypes, with nonamnestic MCI often attributed to non-AD pathologies such as vascular dementia or frontotemporal dementia [55]. We hypothesized that 1) across groups, patients as a whole would show relationships between perseverative errors and other cognitive abilities in a hierarchical manner, 2) consistent with trends in Lamar et al. [26], patients with MCI would exhibit fewer Border errors, but not Semantic or Motor errors on the GST-D in comparison with individuals with AD, and 3) memory performance would be related to perseverative errors in AD.
Methods
ParticipantsParticipants were 34 patients who consented to have their clinical data included in a research database and were seen for neuropsychological examination in a memory disorders clinic within an academic medical center. Demographic characteristics are provided in Table 1. Diagnoses were made based upon the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [56] and resulted from a consensus conference of specialists coordinating patient care, including board certified neurologists, neuropsychologists, and radiologists. No attempt at disease-staging was made. Of the 16 patients in the AD group, one was diagnosed with a mixed AD/vascular etiology, while the remaining 15 were determined to have pure AD. Five of the 18 MCI patients were diagnosed with the nonamnestic subtype, while the other 13 were diagnosed with amnestic MCI.
Patients were administered a neuropsychological battery that included Logical Memory (LM) from the Wechsler Memory Scale – Third Edition [57] to assess verbal memory functioning and the Boston Naming Test [58], as well as a semantic fluency measure, to assess language abilities. EFs were measured with several other tests, some of which have been quantitatively normed to yield percentile descriptors of performance, including the Controlled Word Association Test (COWA; [8]), the Digit Span Backward subtest from the Wechsler Adult Intelligence Scale – Third Edition (WAISIII; [59]), Trail Making Test B (Trails B; [60]), Letter Alternation Test (ABBA), and Clock Drawing to command [61]. In addition, several other frontal/motor measures were administered and scored qualitatively, including the GST-D [26] and the Fist-Edge-Palm Test [11].
GST-D scoring
All patients completed the GST-D under the same administrative and scoring procedures outlined by Lamar et al. [26]. Patients were asked to draw geometric shapes (e.g., a triangle) and simple objects (e.g., a house) or to write the corresponding words (e.g., “triangle” or “house”) in response to commands read sequentially by an examiner. The test is specifically designed to include alternating modes of output in order to elicit perseverative behavior. Responses on the GST were scored in a blinded fashion, such that raters had no knowledge of patient diagnosis. The resulting errors were categorized into six separate subtypes, which were then synthesized into the three hierarchical constructs reported from the Lamar et al. principal components analysis [26]. Descriptions of the composite constructs and corresponding perseveration subtypes are presented in Table 2.
Results
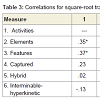
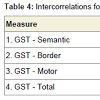
Relationships among cognitive variablesIn order to increase power to detect significant effects, all correlational analyses were conducted across both patient groups combined. In addition, perseveration type data were transformed, using a square-root adjustment, in order to account for nonnormality. GST-D facet scores exhibited modest degrees of shared variance, as indicated with intercorrelations, presented in Table 3. Intercorrelations among the three GST-D indexes, along with total perseverative errors, are shown in Table 4. These data support the hierarchical model of error subtypes inasmuch as Semantic errors, residing at the top of the hypothesized hierarchy of cognitive complexity, share variance with mid-level Border errors (r = .30, p< .05), but not low-level Motor errors, and Border and Motor errors share variance with each other (r = .30, p<.05). This provides support for the notion that Border errors do indeed reside at an intermediate level of complexity between Semantic and Motor subtypes.
Table 5 shows intercorrelations among the three GST indices, total perseverative errors, and select neuropsychological tests. Results indicate that, contrary to Lamar et al. [26], Semantic errors did not correlate significantly with measures of language functioning (the BNT and Animal Fluency), but did correlate significantly with several measures of EFs, including Digit Span Backward (r = -.38, p < .05), Trails B – A (r = .34, p < .05), COWA – FAS (r = -.49, p< .01), Luria Letter Alternation (r = .69, p< .001), and Clock Drawing Errors (r = .42, p< .01). As performance on these neuropsychological tests declined, Semantic perseverations tended to increase. Interestingly, and consistent with our hierarchical model of perseverations, Motor errors did not tend to correlate with these executive tasks, with the exception of Digit Span Backward (r = -.43, p< .01). Additionally, unlike Semantic or Motor errors, the Border error subtype correlated with LM I (r = -.39, p< .05) and LM II (r = -.33, p <.05), suggesting a sensitivity to memory decline across both MCI and AD patients.
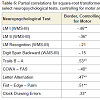
Given the aforementioned evidence suggesting Border error sensitivity to early AD, partial correlations were conducted between Border errors and select neuropsychological tests, while initially controlling for Semantic errors, and then controlling for Motor errors. Results are presented in Table 6. Results suggests that, in comparison with the zero-order correlations presented in Table 4, controlling for Semantic errors tended to decrease the strength of the relationships between Border errors and tests of EFs (i.e., Trails B – A and COWA), while controlling for Motor errors tended to increase the strength of the relationships between Border errors and executive tasks (i.e., Trails B – A, Letter Alternation, and Clock Drawing Errors). These findings provide further evidence of the executive nature of Semantic errors such that, as Semantic error variance is controlled for, the relationship between Border errors and executive tasks is attenuated. In contrast, given that Motor errors lie at the lower end of complexity in the hypothesized cognitive hierarchy, controlling for this variance does not attenuate the strength of the relationship between Border errors and tests of EFs.
Group by perseveration type
In order to test the hypothesis that perseverative errors would be differentially related to progression of cognitive impairment, we conducted a 2-group (AD and MCI) X 3-perseveration type (Semantic errors, Border errors, and Motor errors) mixed ANOVA. The omnibus ANOVA was significant with regard to the main effect of perseverations, F(2, 31) = 13.82, p< .001, partial η2 = .47. Follow-up comparisons to the main effect indicated that Semantic and Motor perseveration errors did not significantly differ across groups, but participants committed significantly fewer Border perseverations in comparison with both Semantic perseverations (p< .01) and Motor perseverations (p< .001). Moreover, and in line with our prediction of a differential relationship between progression of AD and the three perseveration types, we conducted a 2-group X 3-perseveration type planned polynomial comparison. The quadratic effect was significant, F(1, 32) = 5.53, p< .05, partial η2 = .15. Follow-up analyses indicated that, although the three perseveration types did not differ from one another in the AD group, significantly fewer Border errors were committed in comparison with both Semantic errors (p< .01) and Motor errors (p< .001) in the MCI group. These results suggest that individuals with MCI produce significantly fewer Border errors in comparison with Semantic and Motor errors, but as the underlying disease process advances and patients convert to AD, there is an increase in number of Border errors such that Border errors are committed at an equivalent rate compared to Semantic and Motor errors. This effect is presented in Figure 1.
Discussion
The purpose of this study was to investigate perseverative error subtypes and neuropsychological test performance in AD and MCI. We utilized the GST-D [12,26,50] and a battery of standard neuropsychological tests. Results indicate that patients show relationships across perseverative error subtypes and other cognitive abilities in a hierarchical way. Specifically, intercorrelational analyses among GST-D sub-facets demonstrated that the hypothesized second tier of the hierarchy – Border errors – shared variance with both Semantic and Motor errors, which were not significantly correlated with each other. Moreover, Semantic errors, which reside at the top of the theoretical hierarchy, correlated with multiple neuropsychological measures of EFs, while Motor errors, at the lowest level, did not. This finding is partially consistent with Lamar et al., [26], although these investigators reported negative correlations between Semantic errors and performance on measures of language functioning (i.e., the BNT and Animal Fluency) in AD, which were not present in our data. Finally, partial correlations suggested that controlling Semantic error variance weakened the relationships between Border errors and executive tasks, while controlling Motor error variance strengthenedthem.Our hypothesis that GST-D Border errors reflect the progression from MCI to AD was directly supported by a significant quadratic interaction between error subtype and patient group. Our results converge with trends from Lamar et al. [26], in which AD patients produced only mildly reduced Border, compared to Semantic and Motor errors, while healthy controls showed a notable trend in this direction.
Finally, we demonstrated significant relationships between Border errors and Logical Memory immediate and delayed performance, which were not significantly correlated with Semantic or Motor errors. This differential relationship may help explain the sensitivity of Border errors to AD, given that verbal memory measures (e.g., Logical Memory) are widely utilized for assessing the AD symptomatology.
Broadly, perseverative behavior has been defined as a repetition of a behavior that is no longer congruent with task requirements [3] and has been studied in various populations using different tasks. The GST-D appears to be a valuable instrument with which to elicit and measure perseverations in individuals with dementia. Results of two GST-D studies reported significant correlations between perseverations and various executive tasks, including “out of set” responses on the WAIS-R Similarities subtest [62], and constructional errors on a modified Rey-Osterrieth Complex Figure [23]. This suggests that, as a whole, GST-D errors can be conceptualized as resulting from dysexecutive syndromes, although, as our data suggest, some types of perseverations may be contributing to this effect more than others. Moreover, Rusconi et al. presented differential rates of perseverations on a simple circle cancellation task between patients with unilateral neglect and patients with AD such that the former produced significantly more perseverations than the latter [19]. This suggests that, unlike findings in more complex tasks such as the GST-D, AD symptomatology does not induce perseverations on low level, non-executive tasks.
Bilder and Goldberg highlighted the importance of conceptualizing perseverations vertically (i.e., within a neurocognitive hierarchy) [12], as well as horizontally (i.e., across neurocognitive domains). By utilizing a hierarchical model within a single task and measuring cognitive performance in multiple neuropsychological domains, the current investigation provides evidence to support both vertical and horizontal dimensions. Although the vertical component has not been thoroughly investigated in the literature, several other studies (e.g., [28,29], 63) have demonstrated differential relationships between perseveration subtypes and various outcome measures. Ryan et al. measured graphomotor perseverations on the Bender Gestalt task in 15 individuals with AD who were grouped into those who displayed frequent wandering behavior and those who did not, as reported by a primary caregiver [29]. Results indicated that wanderers tended to show more “Type I” and “Type II”, but not more “Type III” perseverations on the task than nonwanderers. Although Type II perseverations do not have a clear analog to GST perseveration subtypes, Type I errors, which were defined as a “perseveration in which elements of a previous design are utilized in a succeeding design when they are not present in the stimulus figure” (p. 210), appear to overlap with GST-D perseveration of Features, a component of Border errors. Moreover, Pekkala et al. utilized Sandson and Albert’s model of perseverative errors and their results suggested that error subtypes were differentially related to the various stages of AD progression [28,64]. Finally, Lamar et al. administered the GST-D to a sample of individuals with dementias of various etiologies and results of a principle components analysis suggested that increased Semantic and Border errors were related to poorer performance on higher-level tasks [27], suggesting that these GST-D sub-facets are both executive in nature. Taken together, evidence from these studies can be interpreted as broadly consistent with the current findings of 1) the hierarchical nature of GST-D sub-facets and 2) the sensitivity of the Border error subtype to AD symptomatology.
Accumulating evidence suggests that dementing pathologies that present initially in the frontal lobes and lead to severe dysexecutive syndromes may manifest with greater degrees of perseverative behavior than AD (e.g., frontotemporal dementia; see [25,31]). However, evidence shows that some early AD pathology does indeed develop in the frontal lobes [65-68] and, furthermore, that certain attentional and executive tasks are very sensitive to this pathology, possibly even above and beyond standard memory measures [44,46,48,69]. Specifically, Balota et al. and Hutchison et al. both utilized a computerized, cued-Stroop task and conceptualized errors on this task as representing a failure in the attentional control system [44,48,70]. Results from both investigations showed that Stroop errors were more sensitive to the progression of early AD pathology than a battery of neuropsychological tests, including memory tests, with the exception of the Selective Reminding Task [71].
Sensitivity of Border errors to progression of AD in the current study reflects overlap between the cognitive underpinnings of GST-D Border errors and Stroop intrusion errors. Specifically, Border errors reflect either a bleed-over effect from previous figure drawings to new drawings (perseveration of Features) or an incongruency between instructions and behavior such that patients produce alphabetic letters when directed to draw geometric figures (Captured perseveration; see [26]). These errors reflect a breakdown in attentional control inasmuch as this construct has been posited to reflect a higherorder cognitive system that mediates flexibility and ingenuity and the deterioration of which leads to an inability to filter out contextinappropriate information [72], ostensibly leading to dysexecutive behavior. Indeed, evidence from Sebastian et al. suggested that perseverations by AD patients on the Brown-Peterson task [30,73,74] reflected a deficiency in the central executive [75], a construct that is closely related to attentional control. Although it could be argued that the Semantic error subtype could also reflect breakdowns in attentional control, our results suggest that Border errors may be more directly mediated by this system. If this is the case, this overlap in variance between the cognitive substrates of Border errors and the attentional control system may have driven the original principle components analysis from Lamar et al. in a population of demented individuals [26].
Beyond theoretical explanation for the present results, it is important to address the clinical implications of such findings. Perseverations do occur in healthy younger and older adults, but at a significantly reduced rate when compared to individuals with brain dysfunction of various etiologies [34]. Moreover, perseverations in individuals with neurodegenerative conditions have been shown to discriminate the degree of white matter alteration in patients with vascular dementia or AD [21] and to discriminate wanderers from nonwanderersin AD [29], thereby demonstrating sensitivity to both neural changes associated with disease progression and behavioral manifestations of these changes. Equally significant is evidence suggesting that even those with early stages of dementia tend to be unaware of their perseverations, making error correction impossible, and ostensibly creating significant functional impairments in these individuals [24]. Although no studies to our knowledge have directly measured the relationship between GST-D perseverative errors and functional outcomes, Belanger et al. [76] utilized the Behavioral Dyscontrol Scale (BDS; [77]), an instrument with significant task overlap in relation to the GST-D, and demonstrated that the BDS not only discriminates between AD and MCI, but it also predicts performance of activities of daily living (ADLs) above and beyond HVLT-R scores. Moreover, Cahn-Weiner, Boyle, and Malloy utilized standard measures of EFs (i.e., Trails B and the Controlled Oral Word Association Test) to predict both performance-based and caregiverrated ADLs [78]. Taken together, these studies suggest that scores from executive measures of behavior, such as the GST-D, are sensitive to the progression of AD pathology and have inherent utility in the prediction of clinically relevant functional outcomes.
There are several limitations to this report. First, we did not have access to healthy older adults from which to create a matched control group. Although this would have allowed us to stage the progression of AD pathology in a more complete manner with respect to GST-D errors, evidence suggests that healthy older adults tend to perseverate at very low rates [34], thereby decreasing the utility of assessing error subtypes in this population. Second, our sample size was relatively small, reducing our power to detect group differences. Finally, our MCI sample was relatively heterogeneous such that it included nonamnestic as well as amnestic subtypes. Consequently, we cannot definitively rule out contributions from non-AD pathologies to our GST-D and neuropsychological data.
We believe that our results contribute to the literature on the cognitive assessment of progressive AD symptomatology, as well as that on the theoretical hierarchical distribution of perseverative errors in individuals with dementing conditions. Future investigations should examine the relationship between GST-D Border errors and the progression of other dementing illnesses. In addition, GST-D error subtypes should be utilized in the prediction of functional outcomes in AD and other neurodegenerative conditions and these perseverations should be measured on a longitudinal, rather than a cross-sectional, basis, in order to more fully elucidate their sensitivity to disease progression.
References
- Goldberg E, Bougakov D (2005) Neuropsychologic assessment of frontal lobe dysfunction. Psychiatr Clin North Am 28: 567-580.
- Espinet SD, Anderson JE, Zelazo PD (2012) N2 amplitude as a neural marker of executive function in young children: An ERP study of children who switch versus perseverate on the dimensional change card sort. Dev Cogn Neurosci 2 Suppl 1: S49-S58.
- Neisser A (1895) Krankenvorstellung (Fall von “asymbolie”). Allgemeine Zeitschrifte fur Psychiatrie 51: 1016-1021
- Butter CM (1969) Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav 4: 163-171.
- Malloy PF, Webster JS, Russell W (1985) Tests of Luria's frontal lobe syndromes. Int J Clin Neuropsychol 7: 88-95.
- Ruchinskas RA, Giuliano AJ (2003) Motor perseveration in geriatric medical patients. Arch Clin Neuropsychol 18: 455-461.
- Hotz G, Helm-Estabrooks N (1995) Perseveration. Part I: a review. Brain Inj 9: 151-159.
- Benton AL, Hamsher K deS, Sivan AB (1994) Multilingual aphasia examination, 3rd Edition (MAE). Psychological Assessment Resources (PAR), Lutz.
- Berg EA (1948) A simple objective technique for measuring flexibility in thinking. J Gen Psychol 39: 15-22.
- Delis DC, Kramer JH, Kaplan E, Ober Thompkins BA (1987) CVLT, California Verbal Learning Test: Adult Version: Manual. Psychological Corporation, San Antonio.
- Luria AR (1966) Higher cortical functions in man. Basic Books, New York.
- Bilder RM, Goldberg E (1987) Motor perseverations in schizophrenia. Arch Clin Neuropsychol 2: 195-214.
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Müller U, et al. (2011) Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D(2/3) receptor agonist. Biol Psychiatry 70: 754-762.
- Son JH, Kuhn J, Keefe KA (2013) Perseverative behavior in rats with methamphetamine-induced neurotoxicity. Neuropharmacology 67: 95-103.
- Woicik PA, Urban C, Alia-Klein N, Henry A, Maloney T, et al. (2011) A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin Card Sorting Test. Neuropsychologia 49: 1660-1669.
- Kleinman JT, DuBois JC, Newhart M, Hillis AE (2013) Disentangling the neuroanatomical correlates of perseveration from unilateral spatial neglect. Behav Neurol 26: 131-138.
- Nys GM, van Zandvoort MJ, van der Worp HB, Kappelle LJ, de Haan EH (2006) Neuropsychological and neuroanatomical correlates of perseverative responses in subacute stroke. Brain 129(Pt 8): 2148-2157.
- Ronchi R, Posteraro L, Fortis P, Bricolo E, Vallar G (2009) Perseveration in left spatial neglect: drawing and cancellation tasks. Cortex 45: 300-312.
- Rusconi ML, Maravita A, Bottini G, Vallar G (2002) Is the intact side really intact? Perseverative responses in patients with unilateral neglect: a productive manifestation. Neuropsychologia 40: 594-604.
- Vallar G, Zilli T, Gandola M, Bottini G (2006) Productive and defective impairments in the neglect syndrome: graphic perseveration, drawing productions and optic prism exposure. Cortex 42: 911-920.
- Cosentino S, Jefferson A, Chute DL, Kaplan E, Libon DJ (2004) Clock drawing errors in dementia: Neuropsychological and neuroanatomical considerations. Cogn Behav Neurol 17: 74-84
- Davis KL, Price CC, Kaplan E, Libon DJ (2002) Error analysis of the nine-word California Verbal Learning Test (CVLT-9) among older adults with and without dementia. Clin Neuropsychol 16: 81-89.
- Freeman RQ, Giovannetti T, Lamar M, Cloud BS, Stern RA et al. (2000) Visuoconstructional problems in dementia: contribution of executive systems functions. Neuropsychology 14: 415-426.
- Giovannetti T, Libon DJ, Hart T (2002) Awareness of naturalistic action errors in dementia. J Int Neuropsychol Soc 8: 633-644.
- Kertesz A, Nadkarni N, Davidson W, Thomas AW (2000) The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 6: 460-468.
- Lamar M, Podell K, Carew TG, Cloud BS, Resh R et al. (1997) Perseverative behavior in Alzheimer's disease and subcortical ischemic vascular dementia. Neuropsychology 11: 523-534.
- Lamar M, Swenson R, Kaplan E, Libon DJ (2004) Characterizing alterations in executive functioning across distinct subtypes of cortical and subcortical dementia. Clin Neuropsychol 18: 22-31.
- Pekkala S, Albert ML, Spiro A 3rd, Erkinjuntti T (2008) Perseveration in Alzheimer’s disease. Dement Geriatr Cogn Disord 25: 109-114.
- Ryan JP, McGowan J, McCaffrey N, Ryan GT, Zandi T (1995) Graphomotor perseveration and wandering in Alzheimer's disease. J Geriatr Psychiatr Neurol 8: 209-212.
- Sebastian MV, Menor J, Elosua MR (2006) Attentional dysfunction of the central executive in AD: evidence from dual task and perseveration errors. Cortex 42: 1015-1020.
- Thompson JC, Stopford CL, Snowden JS, Neary D (2005) Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatr 76: 920-927.
- Caccappolo-van Vliet E, Miozzo M, Marder K, Stern Y (2003) Where do perseverations come from? Neurocase 9: 297-307.
- Hotz G, Helm-Estabrooks N (1995) Perseveration. Part II: A study of perseveration in closed-head injury. Brain Inj 9: 161-172.
- Ramage A, Bayles K, Helm-Estabrooks N, Cruz R (1999) Frequency of perseveration in normal subjects. Brain Lang 66: 329-340.
- Ridderinkhof KR, Span MM, van der Molen MW (2002) Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain Cogn 49: 382-401.
- Allison RS (1966) Perseveration as a sign of diffuse and focal brain damage. I. Br Med J 2: 1027-1032 contd.
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, et al. (1997) Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA 278: 1363-1371.
- Cabrera SM, Chavez CM, Corley SR, Kitto MR, Butt AE (2006) Selective lesions of the nucleus basalis magnocellularis impair cognitive flexibility. Behav Neurosci 120: 298-306.
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, et al. (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66: 1837-1844.
- Grady CL, Grimes AM, Patronas N, Sunderland T, Foster NL (1989) Divided attention, as measured by dichotic speech performance, in dementia of the Alzheimer type. Arch Neurol 46: 317-320.
- Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F (2007) Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology 69: 1859-1867.
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, et al. (2010) Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med 12: 27-43.
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A (1992) Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol 49: 448-452.
- Balota DA, Tse CS, Hutchison KA, Spieler DH, Duchek JM, et al. (2010) Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: the power of errors in Stroop color naming. Psychol Aging 25: 208-218.
- Belleville S, Bherer L, Lepage É, Chertkow H, Gauthier S (2008) Task switching capacities in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychologia 46: 2225-2233.
- Duchek JM, Balota DA, Tse CS, Holtzman DM, Fagan AM, et al. (2009) The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology 23: 746-758.
- Fine EM, Delis DC, Wetter SR, Jacobson MW, Jak AJ, et al. (2008) Cognitive discrepancies versus APOE genotype as predictors of cognitive decline in normal-functioning elderly individuals: a longitudinal study. Am J Geriatr Psychiatry16: 366-374.
- Hutchison KA, Balota DA, Ducheck JM (2010) The utility of Stroop task switching as a marker for early-stage Alzheimer's disease. Psychol Aging 25: 545-559.
- Goldberg E, Tucker D (1979) Motor perseveration and long-term memory for visual forms. J Clin Neuropsychol 1: 273-288.
- Goldberg E (1986) Varieties of perseveration: a comparison of two taxonomies. J Clin Exp Neuropsychol 8: 710-726.
- Diamond A (2013) Executive functions. Annu Rev Psychol 64: 135-168.
- Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: 183-194.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, et al. (2001) Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56: 1133-1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, et al. (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56: 303-308.
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, et al. (2004) Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256: 240-246.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34: 939-944.
- Wechsler D (1997) WMS-III: Wechsler Memory Scale Administration and Scoring Manual. Psychological Corporation, San Antonio.
- Goodglass H, Kaplan E, Barresi B (2000) Boston Diagnostic Aphasia Examination-Third Edition (BDAE-3), Pearson, San Antonio.
- Wechsler D (1997) (WAIS-III): Administration and Scoring Manual: Wechsler Adult Intelligence Scale--Third Edition. Psychological Corporation, San Antonio.
- Reitan RM (1992) Trail making test: Manual for administration and scoring. Reitan Neuropsychology Laboratory, Tucson.
- Goodglass H, Kaplan E, Barresi B (1983) The assessment of aphasia and related disorders (2nd edn), Lea and Febiger, Philadelphia.
- Giovannetti T, Lamar M, Cloud BS, Swenson R, Fein D, et al. (2001) Different underlying mechanisms for deficits in concept formation in dementia. Arch Clin Neuropsychol 16: 547-560.
- Na DL, Adair JC, Kang Y, Chung CS, Lee KH, et al. (1999) Motor perseverative behavior on a line cancellation task. Neurology 52: 1569-1569.
- Sandson J, Albert ML (1984) Varieties of perseveration. Neuropsychologia 22: 715-732.
- Bunce D, Anstey KJ, Christensen H, Dear K, Wen W, et al. (2007) White matter hyperintensities and within-person variability in community-dwelling adults aged 60-64 years. Neuropsychologia 45: 2009-2015.
- Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, et al. (2007) FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer's disease. Brain 130(Pt 10): 2616-2635.
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T (2000) Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol 47: 430-439.
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, et al. (2004) Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound‐B. Ann Neurol 55: 306-319.
- Twamley EW, Ropacki SA, Bondi MW (2006) Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc 12: 707-735.
- Balota DA, Faust, ME (2001) Attention in dementia of the Alzheimer’s type. In: F Boller, CS (Eds) Handbook of Neuropsychology (2nd edn), Elsevier Science 6: 51-80.
- Grober E, Buschke H, Crystal H, Bang S, Dresner R (1988) Screening for dementia by memory testing. Neurology 38: 900-903.
- Faust ME, Balota DA (2007) Inhibition, facilitation, and attentional control in dementia of the Alzheimer's type: The role of unifying principles in cognitive theory development. In: Gorfein DS, MacLeod CM (Eds), Inhibition in Cognition, Washington, DC, US: American Psychological Association, pp. 213-238.
- Peterson LR, Peterson MJ (1959) Short-term retention of individual verbal items. J Exp Psychol 58: 193-198.
- Peterson LR (1966) Short-term memory. Sci Am 215: 90-95.
- Baddeley AD (1986) Working memory. Oxford, Clarendon Press.
- Belanger HG, Wilder-Willis K, Malloy P, Salloway S, Hamman RF, et al. (2005) Assessing motor and cognitive regulation in AD, MCI, and controls using the Behavioral Dyscontrol Scale. Arch Clin Neuropsychol 20: 183-189.
- Grigsby J, Kaye K (1996) Behavioral dyscontrol scale: Manual (2nd Edn) Ward, CO: BDS
- Cahn-Weiner DA, Boyle PA, Malloy PF (2002) Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol 9: 187-191.