Journal of Neurology and Psychology
Download PDF
Research Article
Supratentorial Deep-Seated Intracerebral Hemorrhage: Imaging, Scale, Prognosis
Zeynep Ozozen Ayas1* and Kıyasettin Asil2
- 1Department of Neurology, Eskişehir Yunus Emre Hospital, Eskişehir, Turkey
- 2Department of Radiology, Sakarya University, Sakarya, Turkey
*Address for Correspondence: Zeynep Ozozen Ayas, Department of Neurology, Eskişehir Yunus Emre Hospital, Uluonder av. Salih bozok St. No: 23 Tepebaşı/Eskişehir, Turkey, Tel: 05059039605; E-mail: zozozen@hotmail.com (or) zozozen81@gmail.com
Citation: Ayas ZO, Asil K. Supratentorial Deep-Seated Intracerebral Hemorrhage: Imaging, Scale, Prognosis. J Neurol Psychol. 2018; 6(2): 5.
Copyright © 2018 Ayas ZO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 6, Issue: 2
Submission: June 26, 2018 | Accepted: August 09, 2018 | Published: August 16, 2018
Abstract
Background: Intracerebral hemorrhages (ICH) constituting 12% of all strokes have a significant mortality rate. Supratentorial hemorrhages occur in 90% of the cases, of which 60% constitute deep seat areas (basal ganglia and thalamus). Many markers and scales have been tried to be developed for predicting mortality, especially hematoma volume. In this study, it was investigated that the effects of demografic features, initial symptoms, glaskow coma score (GCS), localization, hematoma volume, internal and external capsule involvement, intraventricular hemorrhage on prognosis and in-hospital mortality in patients with supratentorial deep-seated spontaneous ICH.
Methods: The patients with deep-seated spontaneous intracerebral hematoma who were referred to the hospital were evaluated for age, gender, side of hemisphere, localization, initial symptoms, GCS,hematoma volume (ABCx0.562) (0-10 cc, 10-30 cc, 30-60 cc, >60 cc), internal and external capsule involvement, ventricular involvement, modified rank in scale (MRS) (1-2: independent 3,4,5: dependent, 6: death) and in-hospital mortality were recorded.
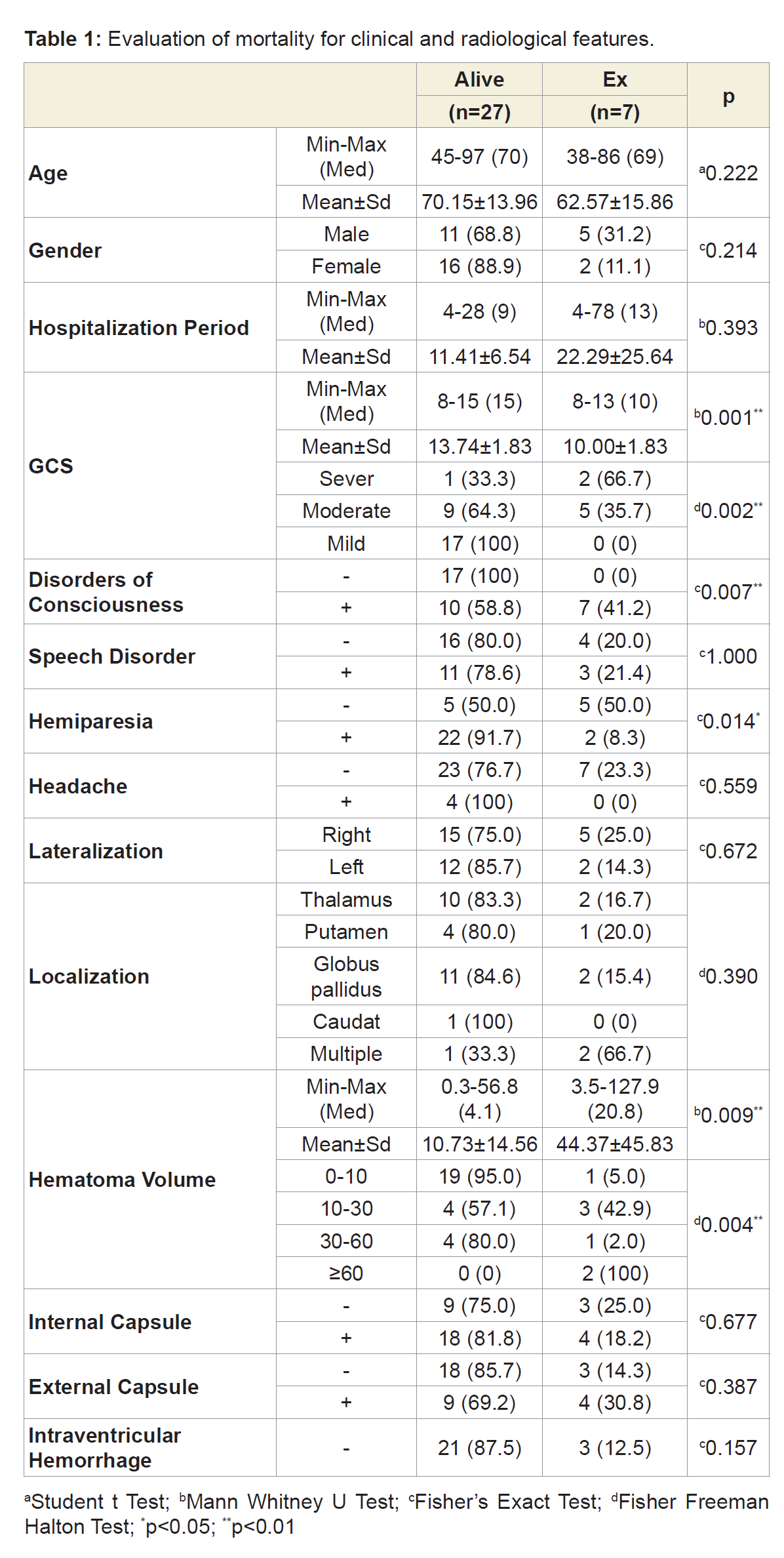
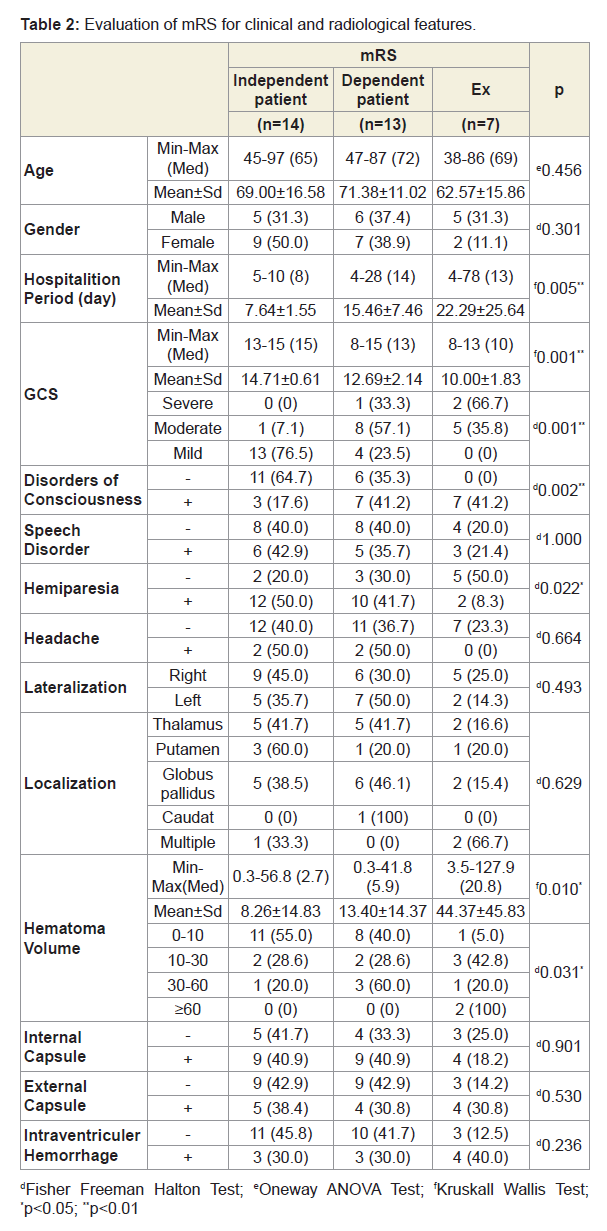
Results: A total of 34 patients (18 female, 16 male) were included in our study. The mean age of the patients was 68.58±14.45 (38-97) years. Mean hospitalization period was 13.64±13.15 (4-78) days.GCS in emergency department was 12.9±2.36 (8-15). Disorders of consciousness were observed in 17 patients (50%), speech disorders in 14 patients (41.1%), motor deficit in 24 patients (70.5%) and headache symptoms in 4 patients (11.7%) on hospital admission. 20 of the hematomas were in the right (58.8%) and 14 were in the left (41.2%) hemisphere. Hematoma localization included globus pallidus in 13 (38.2%) patients, thalamus in 11 (32.3%) patients, putaminal region in 5 (14.7%) patients and coexistent involvement of thalamus-putamenglobus pallidus in 3 (8.8%) patients. Hematoma volumes average were 17.6 cc, 20 (58.8%) patients with 0-10 cc, 7 (20.5%) patients with 10-30 cc, 5 patients with 30-60 cc (14.7%) and 2 patients (5.8%) with over 60 cc were determined. 22 patients (64.7%) with internal capsule involvement, 13 (38.2%) with external capsule involvement and 10 (29.4%) patients with intraventicular hemorrhage were detected. According to MRS, 14 patients (41.2%) were independent, 13 patients were dependent (38.2%), and in-hospital mortality was detected in 7 patients (20.6%). It was found that age, gender, side of hemisphere, speech impairment, headache, localization, internal and external capsule involvement, ventricular involvement parameters did not affect the prognosis and mortality. A significant difference was found between mortality and increased hematoma volume and reduced. (p=0.004, p=0.002, p<0.01) Mortality rates were found to be increased in patients with disorders of consciousness, whereas mortality rates in patients with motor deficit were found to be significantly lower. (p=0.007, p=0.014, p<0.05). A significant difference was found between the hospitalization and the prognosis. (p=0.005; p<0.01). However, no significant difference was found between the duration of hospitalization of the dependent patient group and the patients who died. (P> 0.05).
Conclusion: Hematoma volume, GCS, disorders of consciousness are important parameters as a predictor on prognosis and in-hospital mortality, however further extensive studies are needed to evaluate relationship between mortality and motor deficit as an initial symptom.
Keywords:
Intracerebral hemorrhage; Supratentorial; Imaging; Scale; Prognosis
Introduction
Intracerebral hemorrhage (ICH) strokes have a significant mortality rate: 12% of all strokes [1]. Supratentorial hemorrhages occur in 90% of the cases, of which 60% constitute deep seat areas (basal ganglia and thalamus) [2]. Despite improvements in medical care, ICH strokes are the most fatal form of stroke. According to Trifht, the short-term outcome of patient with ICH is poor [3]. Areisen says that many markers and scales have been developed for predicting mortality, especially hematoma volume [4].The most important models are ICH scales designed by Hemphill et al. and ICH grading scale designed by Ruiz-Sandoval et al. [5,6]. According to Joon, these markers and scales were used mostly in studies involving all localizations [7]. According to Falcone, limited data are available about supratentorial deep-seated spontaneous ICH [8].
The results of this study reveal how supratentorial deep-seated spontaneous ICH is altered by variables such as age, gender, initial symptoms, hospitalization duration, lateralization, glaskow coma score (GCS), localization, hematoma volume, internal and external capsule involvement, and the intraventricular hemorrhage on prognosis. We also investigate the in-hospital mortality in patients. We also discuss the importance of clinical and radiological features that can serve as predictors of a prognosis.
Materials and Methods
Patients who were diagnosed with deep-seated spontaneous intracerebral hematoma in Sakarya University Training and Research University between January 1, 2011-December 31, 2017 were included respectively. We recorded the results of in-hospital mortality in relation to age, gender, mean hospital stay, lateralization, localization, initial symptoms, GCS, hematoma volume, intraventricular hemorrhage, internal and external capsular involvement, and modified rankin scale (mRS). Localization was divided into the two hemispheres: right and left. Patients with supratentorial deep-seated localization were evaluated as globus pallidus, thalamus, putamen, and multiple involvement. The ABC/2 method was used for determining hematoma volume. The distance between the anteroposterior end points was determined as A (length) and the distance between the lateromedial end points was determined as B (width) in centimeters (cm). The hemispherical cross-sectional size was multiplied by C dimension (height) and by the formula AxBxCx0.562. In addition, hematoma volumes were divided into 4 groups, as the following: 0-10 cm3, 11-30 cm3, >31-60 cm3, and 61 cm3. Points 1 and 2 in the mRS can independently manage the patient’s life. Points 3, 4, and 5 manage the patient’s dependency. Point 6 manages death.
The software Statistical Package for Social Sciences (SPSS) for Windows 22.0 was used for all statistical analyses. The descriptive statistics included the mean value and SD. The quantitative variables were compared using the following tests: Student’s T, Mann Whitney-U, Kruskal-Wallis, Fisher, and Wilcoxon Signed-Rank. The qualitative variables were compared with the χ2 (Chi Square) test. Spearman’s Rho Correlation test was used to examine correlations between study parameters. The results were interpreted at a confidence value of 95% and at a significance value of p<0.05.
Results
A total of 34 patients (18 female, 16 male) were included in the study. The mean age of the patients was 68.58±14.45 (38-97) years. Mean hospitalization period was 13.64±13.15 (4-78) days. GCS in emergency department was 12.9±2.36 (8-15). Upon admittance into the hospital, disorders of consciousness were observed in 17 patients (50%), speech disorders in 14 patients (41.1%), motor deficit in 24 patients (70.5%) and headache symptoms in 4 patients (11.7%). 20 of the hematomas were in the right (58.8%) and 14 were in the left hemisphere (41.2%). Hematoma localization included globus pallidus in 13 patients (38.2%), thalamus in 11 patients (32.3%), putaminal region in 5 patients (14.7%), and coexistent involvement of thalamusputamen-globus pallidus in 3 patients (8.8%). The mean hematoma volume was 17.6 cc; specifically, the study found20 patients (58.8%) with 0-10 cc,7 patients (20.5%) with 10-30 cc, 5 patients (14.7%) with 30-60 cc, and 2 patients (5.8%) with over 60 cc. Furthermore, the study found 22 patients (64.7%) with internal capsule involvement, 13 patients (38.2%) with external capsule involvement, and 10 patients (29.4%) with intraventricular hemorrhage. According to the mRS, 14 patients (41.2%) were independent, 13 patients (38.2%) were dependent, and 7 patients (20.6%) were in-hospital mortalities. The research reveals that age, gender, hemisphere, speech impairment, headache, localization, internal and external capsule involvement, and intraventricular hemorrhage parameters did not affect the prognosis and mortality. A significant difference was found between mortality and increased hematoma volume and reduced GCS (p = 0.004, p = 0.002, p <0.01) (Table 1). Mortality rates were found to increase in patients with disorders of consciousness, whereas mortality rates in patients with motor deficit were found to be significantly lower (p=0.007, p=0.014, p<0.05) (Table 1). A significant difference was found between the hospitalization duration and the prognosis (p=0,005; p<0,01) (Table 2). However, no significant difference was found between the duration of hospitalization of the dependent patient group and the patients who died (p>0.05).
Discussion
There are some studies in the literature about ICH but these studies mostly investigated all localizations. Our study is an important contribution in terms of the investigation of only supratentorial deepseated hematomas.
Most individuals with ICH are 55 or older [9]. In our study, the mean age of the patients was 68.58±14.45 (38-97) and it was determined that elderly age had no effect on prognosis and mortality. On the other hand, another study reported that patients over 65 years of age had a worse prognosis [10]. No significant gender difference was found in our study (18 F, 16 M). It has been reported that male gender ratio is seen more in the literature [10,11]. In our study, it was determined that gender had no effect on prognosis and mortality. Our conclusion is confirmed by another study that evaluated all patients with an intracerebral hemorrhage. The study reported that there was no gender difference in mortality [12]. The study also reported that the female gender is associated with a better prognosis [13].
Clinicians may encounter a wide clinical spectrum of contralateral motor and sensory deficits in supratentorial ICH of caudate, thalamus, and putamen involvement. In addition, aphasia, neglect, gaze deviations, and hemianopsia occur due to the damage of the connective fibers in the subcortical white matter [14]. In our study, the most common hospital initial symptoms were the following: motor deficits in 24 patients (70.5%), disorders of consciousness in 17 patients (50%), speech impairment in 14 patients (41.1%), and headache in 4 patients. In our study, mortality was increased in cases with disorders of consciousness, but the mortality rate was found to be significantly lower in patients with a motor deficit. It has been reported that the initial state of consciousness of the patient is important for prognosis [15]. Similarly, in a study of 166 patients, it was determined that the state of consciousness at the time of admission was particularly important in prognosis [16]. The first GCS in thalamic hemorrhages has been reported to be an important predictive factor for prognosis [17]. In our study, it was thought that the mortality rate in patients with a motor deficit may be related to the limited number of patients.
Although right-hemisphere lateralization accounted for 58.8% of our study, lateralization had no effect on prognosis and mortality. In a similar study, it was determined that there was no effect on the mortality of hematoma lateralization [18].
Different rates have been reported in terms of hemorrhage localizations.In a study that examined 104 patients, 41% lober, 36% putamen and 16% thalamus localizations were detected [15]. In our study, we observed 38.2% globus pallidus, 32.3% thalamus, 14.7% putamen and 8.8% multiple involvement. In another study, 84 basalganglia and 57 thalamic hematomas were detected [10].
Hematoma volume is the most important determinant of inhospital mortality [19]. It is also an independent factor in determining the clinical prognosis [20]. In our study, however, a significant difference was detected between hematoma volume and mortality. In one study for example, it was determined that the mortality rate increased by 1% when the hematoma volume increased by 1 cm3 [21]. In another study, significant mortality was observed in hematomas larger than 30 cm, which affected the prognosis volume [15]. In the case of putaminal hemorrhage, hematoma volumes were found to be statistically-and significantly-higher in patients who died [18]. However, there was no significant difference between living and dead patients with thalamic localized hematoma volumes [18].
It has been reported that all intracerebral hemorrhage with ventricular involvement suffer both poor prognosis and increased mortality [10,13,15]. Similarly, the mortality rate of patients who had ventricular involvement was found to be higher than those who did not [17]. However, ventricular involvement was not found to have any effect on prognosis and mortality in patients with a supratentorial deep-seated hemorrhage. Since the hemorrhage in the thalamus and caudate localization can be easily spreaded to ventricle, it is thought that it does not affect prognosis. However, ventricular involvement in putaminal hemorrhage has been reported to alter the prognosis [22].
The mean hospitalization period of our patients was 13.64±13.15 (4-78) days. According to mRS, 14 patients (41.2%) were independent, 13 patients (38.2%) were dependent, and in-hospital mortality was 7 patients (20.6%). A significant difference was found between the hospitalization and the prognosis. However, no significant difference was found between the duration of hospitalization of the dependent patient group and the patients who died. It is thought that possible triggers of this significant difference are the increased intensive care and the increased complications associated with the long-term hospitalization of patients with mortality or dependence. In a study about stroke patients, hospitalization duration in an intensive care unit was detected to correlate with the mortality rate [23].
In a recent study, the intracerebral hemorrhage score (ICH Score), when calculated with age, localization, hematoma volume, ventricular involvement, and GCS, was not considered successful in predicting mortality [24]. It has been emphasized that these should be reevaluated to make it more helpful to predict prognosis. A partial reevaluation does occur in another recent study, which attempts a revision of the score system through different definitions: the ICH Score FS instead of GCS [25]. Although there are known parameters, significant predictive values are needed for the prognosis and mortality of patients with ICH.
Intracerebral hemorrhage treatment has evolved over the past decade in the setting of increasing knowledge about risk factors, pathophysiology, and management. Early airway protection, control of malignant hypertension, urgent reversal of coagulopathy, and surgical intervention may increase the chance of survival for patients with severe ICH. Intensive lowering of systolic blood pressure to <140 mm Hg is proven safe by two recent randomized trials. Transfusion of platelets in patients on antiplatelet therapy is not indicated unless the patient is scheduled for surgical evacuation of hematoma. According to Dastur, for patients with large ICH (volume>30 cubiccentimeter) or symptomatic perihaematoma oedema, it may be beneficial to keep serum sodium level at 140-150 mEq/L for 7-10 days to minimize oedema expansion and the mass effect [26].
The role of surgery in the management of ICH is still contradictive. According to Akhigbe, there is insufficient evidence to justify a general policy of early surgery for patients with ICH compared to initial medical therapy [27]. However, another study by Yilmaz showed that surgical treatment is very important to achieve successful outcomes in a select group of patients with supratentorial ICH [28]. At this point, the predictors are important especially to decide surgical approachment in treatment of ICH.
According to Dastur, there is no benefit for seizure prophylaxis or aggressive management of fever or hyperglycemia. Early aggressive comprehensive care may improve survival and functional recovery [26].
Additionally, in a recent study Wang shows that the transtemporal approach of placing intracranial pressure sensors into the temporal horn of the lateral ventricle to manage the spontaneous supratentorial intracerebral hemorrhage broken into ventricles is a safe, effective, and less complicated treatment measure [29].
Hematoma volume, GCS, and disorders of consciousness are important parameters as a predictor on prognosis and in-hospital mortality, however further extensive studies are needed to evaluate the relationship between mortality and motor deficit as an initial symptom.
References
- Keir SL, Wardlaw JM, Warlow CP (2002) Stroke epidemiolology studies have underestimated the frequency of intracerebral haemorrhage. A systematic review of imaging in epidemiological studies. J Neurol 249: 1226-1231.
- Warlow C, vanGijn J, Dennis M, Wardlaw J, Bamford J, et al. (2008) Stroke cerebral arterial supply, (3rd edn). Blackwell Publishing Group, pp. 438
- Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA (2001) Incidence of the major stroke subtypes: initial findings from the North East Melbourne stroke incidence study (NEMESIS). Stroke. 32: 1732-1738.
- Ariesen MJ, Algra A, van der Worp HB, Rinkel GJ (2005) Applicability and relevance of models that predict short term outcome after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 76: 839-844.
- Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32: 891-897.
- Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Padilla-Martinez JJ, Gonzalez-Cornejo S (2007) Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke 38: 1641-1644.
- An SJ, Kim TJ, Yoon BW (2017) Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 19: 3-10.
- Falcone GJ, Biff A, Brouwers HB, Anderson CD, Battey TW, et al. (2013) Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol 70: 988-994.
- Beslac-Bumbasirevic L, Paden V, Jovanovic DR, Stefanovic-Budimkic M (2012) Spontaneous intracerebral hemorrhage. Periodicum Biologorum 114: 337-345.
- Tekinarslan I, Guler S, Utku U (2012) Spontaneous intracerebral hemorrhage: etiology and yearly prognostic factors. Turk J Neurol 18: 88-95.
- Ariesen MJ, Claus SP, Rinkel GJ, Algra A (2003) Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 34: 2060-2065.
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, et al. (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage overtime, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9: 167-176.
- Castellanos M, Leira R, Tejada J, Gil-Peralta A, Davalos A, et al. (2005) Predictor of good outcome in medium to large spontaneous supratentorial intracerebral hemorrhages. J Neurol Neurosurg Psychiatry 76: 691-695.
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, et al. (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344: 1450-1460.
- Eroglu A, Atabey C, Topuz AK, Çolak A, Demircan MN (2012) Evaluation of 104 cases spontaneous intracerebral hematoma. Turk Neurosurg 22: 167-170.
- Daverat P, Castel JP, Dartigues JF, Orgogozo JM (1991) Death and functional outcome after spontaneous intracerebral hemorrhage: a prospective study of 166 cases using multivariate analysis. Stroke 22: 1-6.
- Lee SH, Park KJ, Kang SH, Jung YG, Park JY, et al. (2015) Prognostic factors of clinical outcomes in patients with spontaneous thalamic hemorrhage. Med Sci Monit 21: 2638-2646.
- Kutluhan S, Fidan H, Akhan G (2000) Relationship between CT findings and case fatality rates in intracerebral hemorrhage. Turk J Cerebrovasc Dis 6: 81-85.
- Yildiz OK, Arsava EM, Akpinar E, Topcuoglu MA (2011) Hematoma volume as a sole predictor of in-hospital mortality following spontaneous intracerebral. Turk J Cerebrovascr Dis 17: 63-66.
- Leira R, Davalos A, Silva Y, Gil-Peralta A, Tejada J, et al. (2004) Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 63: 461-467.
- Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, et al. (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66: 1175-1181.
- Kase CS, Mohr JP, Caplan LR (1992) Intracerebral hemorrhage. In: Stroke: pathophysiology, diagnosis and management, (2ndedn). Churchill-Livingstone Inc.
- Arslan Z, Ozmen O, Comez M (2016) Factors affecting the mortality of the patients with ischemic stroke in reanimation intensive care unit. Med J Mustafa Kemal Univ 7: 23-31.
- McCracken DJ, Lovasik BP, McCranken CE, Frerich JM, McDougal ME, et al. (2018) The intracerebral hemorrhage score: a self-fulfilling prophecy? Neurosurgery.
- Braksick SA, Hemphill JC 3rd, Mandrekar J, Wijdicks EF, Fugate JE (2018) Application of the FOUR score in intracerebral hemorrhage risk analysis. J Stroke Cerebrovasc Dis 27: 1565-1569.
- Dastur CK, Yu W (2017) Current management of spontaneous intracerebral haemorrhage. Stroke Vasc Neurol 2: 21-29.
- Akhigbe T, Zolnouurian A (2017) Role of surgery in the management of the patients with supratentorial spontaneous intracerebral hematoma: critical appraisal of evidence. J Clin Neurosci 39: 35-38.
- Yilmaz C, Kabatas S, Gulsen S, Cansever T, Gurkanlar D, et al. (2010) Spontaneous supratentorial intracerebral hemorrhage: does surgery benefit comatose patients? Ann Indian Acad Neurol 13:184-187.
- Wang F, Yang T, Niu C (2018) The effect of transtemperal approach and placement of intracranial pressure sensor into temporal horn of lateral ventricle in management of spontaneous supratentorial intracerebral hemorrhage broken into ventricles. J Craniofac Surg.