Journal of Neurology and Psychology
Download PDF
Research Article
*Address for Correspondence: Alexander M. Ponizovsky, MD, PhD, Research Unit, Mental Health Services, Ministry of Health, 39 Yirmiyahu St., PO Box 1176, Jerusalem 9446724, Israel, E-mail: alexpon8@gmail.com
Citation: Ponizovsky AM, Barshtein G, Nechamkin Y, Lecht S, Bergelson LD. Differences in the Lipid Domain Organization of Erythrocyte Membranes in Patients with Schizophrenia. J Neurol Psychol. 2015;3(1): 6.
Copyright © 2014 Ponizovsky Am, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 3, Issue: 1
Submission: 24 November 2014 | Accepted: 22 December 2014 | Published: 29 December 2014
Moreover, the higher GP values of Laurdan obtained with RBC samples from patients with NS (Figure 1B) are indicative of reduced water content (water permeability) of the red cell membrane in NS schizophrenia [35]. The above differences could be ascribed simply to higher levels of SM [9,38] and/or elevated cholesterol [11,39] in the patient samples, but they also might be associated with differences in the interaction of various lipids with the membranes’ proteins. To visualize such differences we measured the resonance energy transfer (RET) from tryptophans to fluorescent labeled phospholipids [40].
In the past two alternative hypotheses regarding a possible role of PGE1 in the etiology of schizophrenia were formulated (reviewed in [16]). According to one hypothesis [45] schizophrenia may be associated with excess in prostaglandins including PGE1, whereas the other suggests that the disease is related to PGE1 deficiency [46,47] probably caused by a defect in the metabolism of precursor fatty acids. The data of Figure 2 suggest that the membrane may make up for such deficiency by higher sensitivity. Summarizing, the results of this preliminary study suggest the existence of differences in the membrane lipid organization and in its response to the action of PGE1 in RBCs from a subgroup of patients with NS-schizophrenia as compared to red cells from patients with PS-schizophrenia and healthy donors. At the moment these data are subject to several important limitations. Thus, all patients were medicated, the number of patients and controls is small, and effects of age, gender, smoking and diet still have not been explored. Moreover, effects in buffer solution may differ from those in plasma, and although evidence to support the use of RBC as a model to study lipid pathology in schizophrenia exists [48,49], the relevance of that model to events in the central nervous system is still not clear. Finally, we did not measure cognitive function in the patients, and it is very possible that the between-group differences in the lipid domain organization of erythrocyte membranes in schizophrenia could be associated with (or attributed to) the possible differences in cognitive deficits rather than with the positive-negative symptom dichotomy. This assumption needs further investigation. Nevertheless, the result of the present study is in line with numerous previous findings, which demonstrated substantial deviations in the biochemical and biophysical state of erythrocytes in schizophrenia (reviewed in [50]). Noticeable, the protein composition of erythrocytes from schizophrenia patients was found not to differ from that of normal red cells [51]. It is thus possible that the primary cause of the observed changes may be some defect in lipid metabolism (see [29,52,53] and the literature cited therein) leading to changes in the lipid domain organization which in turn influence the properties and surface distribution of the red cell proteins. Further studies of the lipid domain organization in peripheral and neuronal cells may prove helpful to achieve a better understanding of the role of lipids in the development of the specific subtypes of schizophrenia.
Differences in the Lipid Domain Organization of Erythrocyte Membranes in Patients with Schizophrenia
Alexander M. Ponizovsky1*, Gregory Barshtein2, Yakov Nechamkin3, Shimon Lecht4 and Lev D. Bergelson4
- 1Research Unit, Mental Health Services, Ministry of Health, Jerusalem, Israel
- 2Department of Biochemistry, Hadassah Medical School, The Hebrew University of Jerusalem, Israel
- 3Sha’ar Menashe Mental Health Center, Hadera, Israel
- 4Biological Membrane Group, School of Pharmacy, the Hebrew University of Jerusalem, Israel
*Address for Correspondence: Alexander M. Ponizovsky, MD, PhD, Research Unit, Mental Health Services, Ministry of Health, 39 Yirmiyahu St., PO Box 1176, Jerusalem 9446724, Israel, E-mail: alexpon8@gmail.com
Citation: Ponizovsky AM, Barshtein G, Nechamkin Y, Lecht S, Bergelson LD. Differences in the Lipid Domain Organization of Erythrocyte Membranes in Patients with Schizophrenia. J Neurol Psychol. 2015;3(1): 6.
Copyright © 2014 Ponizovsky Am, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 3, Issue: 1
Submission: 24 November 2014 | Accepted: 22 December 2014 | Published: 29 December 2014
Abstract
Gradually accumulating evidence indicates that schizophrenia may be accompanied by alterations of membrane phospholipids, however knowledge about how it affects lipid domain organization of membrane is lacking. We compared the membrane lipid domain organization of red blood cell (RBC) membranes from two groups of patients with schizophrenia selected by their high positive syndrome (PS) or negative syndrome (NS) scores on the Positive and Negative Syndrome Scale, and control subjects without known psychiatric history. Differences in the surface distribution of the membrane phospholipids were elucidated by: 1) registering the fluorescence excitation spectra of the polarity-sensitive lipid probe Laurdan incorporated into the RBC membrane, 2) measuring the resonance energy transfer (RET) from tryptophan to fluorescent analogs of the main RBC phospholipids phosphatidylcholine and sphingomyelin, and 3) determining the changes in RET from tryptophan to the sphingomyelin probe induced by prostaglandin E1. The data obtained with RBCs from either normal subjects or PS patients were largely similar, but differed significantly from those obtained with RBCs of patients with NS schizophrenia. The results support validity of the clinical distinction of schizophrenia into positive and negative subtypes.Keywords
Erythrocyte membrane; Lipid domains; Fluorescence measurements; Schizophrenia; SymptomatologyIntroduction
Most approaches to subtyping schizophrenia have made use of the concept of positive and negative syndromes (PS and NS). PS are characterized by hallucinations, delusions and thought disorders whereas apathy, emotional blunting and psychomotor retardation are regarded as NS [1]. Although the positive-negative dichotomy is believed by some authors to be a simplification [2-4], it has important clinical and prognostic significance. A number of recent investigations have revealed that the positive and negative syndromes are associated with different biochemical changes. Particularly, previous studies have detected certain specific lipid aberrations in red blood cells (RBC) from psychiatric patients with predominantly negative symptoms [5-8]. Recently, we reported on noticeable increases in both the sphingomyelin level and the aggregability of RBCs from patients with schizophrenia with mainly NS in comparison to those from patients with schizophrenia with mainly PS and normal controls [9,10]. Previously, RBCs from patients with schizophrenia were found to be enriched also in cholesterol and glycolipids [11-13] and their membranes were reported to be less “fluid” than those of normal erythrocytes [11,13]. However the concept of membrane fluidity depends on the assumption that the hydrophobic region of the membrane is structurally and dynamically homogeneous, whereas in eukaryotic cells including human and rabbit erythrocytes [14-18], the surface lipids appear to distribute unevenly between lateral micro-domains. Since the activity of membrane receptors and membrane bound enzymes depends on the physical state of their nearest environment, local properties pertaining to lipid domains in the immediate neighborhood of membrane proteins may be more relevant than bulk lipid properties. Nevertheless the connection between lipid domain structure and pathophysiological events is a new, still poorly explored field of clinical biochemistry. In recent years it has been found that in many cells sphingolipids and cholesterol cluster together forming movable domains or rafts, which are believed to be involved in signal transduction and cell-cell interactions [19]. In view of the unusually high sphingomyelin (SM) levels in RBC from patients with NS schizophrenia [9] we therefore thought it of interest to obtain more information about the lipid distribution in the RBC membrane, particularly, the lateral segregation of its two main phospholipid species, SM and phosphatidylcholine (PC) in membranes of RBCs isolated from the blood of patients with schizophrenia with defined clinical symptom scale scores. In this study we attempted to detect differences in the lipid domain structure of erythrocytes from normal subjects and subgroups of patients with patients with PS or NS schizophreniaby studying excitation spectra of the polarity-sensitive fluorescent membrane probe Laurdan, as well as by measuring the fluorescence resonance energy transfer (RET) from protein tryptophans to fluorescent analogs of SM and PC incorporated into the erythrocyte membrane.Some earlier studies of polyunsaturated fatty acids, prostaglandin synthesis and prostaglandin receptor activity suggested a role for prostaglandins in the pathophysiology of schizophrenia (reviewed in [20]). Because red blood cells are known to be extremely sensitive to prostaglandin E1 (PGE1) [15,21-26] (see also [21,22] for reviews of the older literature), we here use the multilipid probe approach also to detect possible differences between the response to PGE1 of the phospholipid domain organization in the membranes of normal red cells and those isolated from the blood of patients with predominantly NS schizophrenia.
Methods
SubjectsThe study group included 32 patients hospitalized in acute and open wards at the Sha’ar Menashe Medical Heath Center (mean age = 42.1 years, SD = 9.2; 16 men and 16 women). All patients met the criteria for DSM-IV diagnosis of schizophrenia [27]: 13 presented with paranoid type (295.30) and 19 with residual type (295.60). The patients were treated with haloperidol 10-20 mg/day or risperidone 6-8 mg/day in unchanged manner at least during 3 months prior to examination and had the same dietary regime. During the screening phase, 9 patients were approached but not enrolled because of systemic chronic medical (n=2), neurological disease (n=1), substance and alcohol abuse/dependence (n=3) and clinically significant abnormalities in hematological and/or in biochemistry screening tests (n=3). The mean age at onset of psychotic symptoms was 23.3 years (SD = 5.2), mean duration of the mental illness was 19.3 years (SD = 11.3), and mean duration of lifetime psychiatric hospitalization was 6.9 years (SD = 8.2). All patients provided written informed consent for the study protocol as approved by the institutional review board and the ethics committee of the Ministry of Health.
The Structured Clinical Interview for DSM-IV Axis I Disorders, Patients Edition [27] was used for diagnosis. The severity of illness, positive and negative symptoms was assessed by two experienced clinicians (A.M.P. and Y.N.) specially trained in using the Positive and Negative Syndrome Scale (PANSS) [28]. The total mean PANSS score was 96.2 (SD = 16.5), Positive Syndrome Scale mean score was 21.5 (SD = 6.9), and Negative Syndrome Scale mean score was 30.7 (SD = 10.2). According to the PANSS total scores after a 3-month treatment with antipsychotics, the patients were considered as a treatment-resistant group.
Since in previous studies it has been shown that RBC-membrane biochemical abnormalities are differentially associated with the positive and negative symptoms of schizophrenia [9,29], out of the entire sample we selected for investigation only patients who had maximal high scores on the Positive Syndrome Scale (≥30) and Negative Syndrome Scale (≥40). The selected subgroups (n=19) did not differ significantly from the entire sample in characteristics other than the severity of positive and negative symptomatology. Control blood samples were obtained at the Hadassah Medical Hospital, Jerusalem, from 10 healthy donors (5 men and 5 women) with mean age = 41.1 years (SD = 9.3), without known psychiatric history.
Fluorescent probes
The fluorescent probes Antrylvinyl-labeled Sphingomyelin (ASM) and Antrylvinyl-labeled Phosphatidylcholine (APC) were synthesized according to Molotkovsky et al. in Shemyakin Institute of Bioorganic Chemistry, Moscow, and Avanti Polar Lipids Inc., Alabaster, AL, respectively [30,31]. Laurdan was obtained from Molecular Probes, Eugene, USA. Prostaglandins E1 and F2α were purchased from Biomol, Plymouth Meeting, USA.
Preparation and fluorescent labeling of RBC membranes
Erythrocyte ghosts were prepared as described by Barshtein et al. [32]. After lysis the RBC membranes were washed 6 times with Tris buffer and pelleted at 10 000 rpm for 15 min. The phopholipid probes ASM and APC were dissolved in ethanol to a final concentration of 6 μM. Aliquots of these stock solutions were added to suspensions of RBC ghosts in Tris buffer to a final probe-to-phospholipid molar ratio of 1:200 and incubated for 2 h at 36.5 °C. Probe incorporation was controlled by following the relief from self-quenching, i.e. by measuring the increase in intensity of the 430 nm emission upon excitation at 370 nm. For incorporation of Laurdan, 1 μl of 0.25 mM Laurdan solution in dimethylsulfoxide was incubated for 2h at 25 °C with 5 ml of the RBC ghost suspension in Tris buffer at a concentration corresponding to 106 cells/ml according to the procedure described by Barshtein et al. [32]. After incubation the suspensions were centrifuged, and the pellet was re-suspended in 1.5 ml of Tris buffer.
Fluorescence measurements
Fluorescence Spectra were recorded with a Jesco FP 770 spectrofluorimeter. Spectra were collected by using excitation and emission slit widths of 3 nm and 5 nm, respectively. Quenching of tryptophan fluorescence (excited at 295 nm) by ASM or APC in RBC membranes was determined by measuring the decrease in fluorescence intensity of the tryptophan emission (330 nm) induced by the lipid probe. This decrease in fluorescence intensity was expressed as a mean score for each group studied and two-tailed t-tests (Bonferroni-corrected) were performed to compare betweengroup differences in mean scores and standard deviations. The level of test significance was defined as p < 0.001.
Generalized polarization (GP) of Laurdan was calculated according to the equation: GP = (B – R)/(B + R), where B (blue) and R (red) are the two excitation maximum at lower and higher wavelength, respectively [33].
Results and Discussion
Fluorescence measures with Laurdan, ASM and APCThe spectral properties of Laurdan [33-35] and the anthrylvinyllabeled phospholipids ASM and APC [36] and their use in studies of lipid domains in artificial and natural membranes [32,33,37] have been described. Laurdan spectra are extremely sensitive to the polarity and phase state of the probe’s environment. When Laurdan is inserted into phospholipid membranes its excitation spectrum usually displays two incompletely resolved peaks: a “red” one at about 390 nm which is particularly intense in the gel phase, and a “blue” peak at about 350 nm showing maximal intensity in liquid crystalline phases [34,35]. In liquid crystalline environment both bands are observed in the Laurdan excitation spectra, but usually maximal excitation is seen in the blue band region [34,35]. The spectra of Laurdan incorporated into membranes of RBCs from normal donors and patients with schizophrenia showed two partly resolved peaks, (Figure 1A), however in the case of normal subjects the “blue” (liquid-crystalline) peak was of higher intensity than the “red” (gel phase) maximum, whereas the opposite was true for RBC isolated from the blood of the patients with schizophrenia with NS included into the present study. The Laurdan Spectra of RBCs from most of the patients with schizophrenia with PS were more similar to those obtained with normal erythrocytes with the higher maximum in the blue region (not shown). Since the affinity of Laurdan for proteins has been shown to be very low, this difference can be interpreted as showing that in RBCs from patients with NS the probe is surrounded by more gel phase phospholipid than in red cells from patients with PS and normal subjects.
Figure 1: A) Excitation spectra of Laurdan incorporated into RBC membranes from 12 schizophrenia patients with severe negative symptoms (NS scores > 40) and 10 normal control subjects. B) General polarization (GP) versus wavelength plots derived from the Laurdan excitation spectra shown in Figure 1A.
Resonance energy transfer
This approach relies upon interaction between two fluorophores, occurring when the emission of one, the energy donor, overlaps with the excitation wavelength of the second, the energy acceptor, and the two fluorophores are in close spatial proximity. Under such conditions the energy absorbed by the donor can be transferred to the acceptor, which will then fluoresce as though it had been excited directly, whereas the emission of the donor will be less intense than in absence of the acceptor. Since the efficiency of energy transfer depends strongly on the distance between the donor and acceptor fluorophores, RET is a sensitive tool permitting the detection of changes in the spatial separation of fluorescent donor and acceptor molecules within a given membrane. In the present study we attempted to visualize such changes in the membranes of RBCs from schizophrenia patients and normal subjects by measuring the energy transfer from tryptophans to two phospholipid probes, ASM and APC, incorporated into the membrane. These probes are close analogs of SM and PC, the two major phospholipids of the erythrocyte membrane; they resemble them closely in their physical properties and were shown to be highly sensitive to separation of lateral domains on the surface of cells, viruses and serum lipoproteins [16,37,40,41].
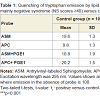
As reported previously [32], ASM or APC separately incorporated into membranes of normal RBC and excited at 370 nm (the wavelength of the excitation maximum of the anthrylvinyl group) showed similar emission spectra with almost identical intensity of the maxima in the 430-440 nm region. However, if ASM or APC were excited at 295 nm (the wavelength corresponding to the excitation maximum of tryptophan), the intensity of the 430-440 nm emission peak of the ASM samples was higher, whereas the tryptophan emission in the 330-340 nm region was considerably weaker than in the fluorescent spectra of ghosts labeled with APC (Table 1). Thus, in agreement with our previous results [32], the tryptophan-to-lipid probe energy transfer was more efficient to the SM probe ASM than to the analog of phosphatidylcholine APC indicating a certain kind of phase separation between the parent lipids in the membrane of normal red cells.
When comparing RET efficiency in ASM or APC labeled RBC membranes from normal subjects and patients with schizophrenia which contain different amounts of PC and SM, it is feasible to discuss the fluorescence intensity changes of the donor tryptophans rather than those of the phospholipid acceptors because the RET-induced fluorescence intensity increase of the lipid probes may be obscured by self-quenching. As shown in Table 1, in membranes of RBC from patients with NS and PS, as well as in those of normal red cells, ASM also quenched the tryptophan fluorescence more efficiently than APC. However, in the NS patients’ samples the quenching effect of the SM probe ASM was much stronger than in erythrocytes from PS patients and healthy subjects. At the same time the quenching effect of the PC probe APC was similar in RBC membranes obtained from NS and PS patients and normal donors. Given that the probes ASM and APC reflect the behavior of their natural counterparts, these data can be interpreted as showing different surface distribution of SM and PC in membranes of red cells from a subgroup of patients with predominantly negative symptoms in comparison to normal controls and PS patients.
Prostaglandin E1 (PGE1) impact
Further differences were detected by comparing the changes of tryptophan-to-probe RET in membranes of RBC from normal subjects and patients with NS schizophrenia induced by exposure of the ghosts to PGE1. For more than 30 years it was known that even sub-physiological concentrations of some prostaglandin induce dramatic changes in the properties of human erythrocytes [21-26], and a specific PGE1 receptor on their membrane has been identified [42-44].
As shown in Table 1, addition of PGE1 to APC- or ASM-labeled erythrocyte membranes caused the efficiency of energy transfer from tryptophans to the two probes (measured as the extent of quenching of the donor fluorescence excited at 295 nm) to shift in different directions: with the SM probe ASM the prostaglandin induced a decrease of RET from tryptophans, whereas the opposite was true for the PC probe APC. The dependence of the tryptophan fluorescence quenching by ASM on the concentration of PGE1 in membranes of red cells from patients with NS-schizophrenia and normal donors is shown in Figure 2. In both cases maximal effects were observed at about 10-9 M PGE1. At such concentration the effect of PGE1 was considerably higher in the patients group than in the controls. No effect was seen in the presence of PGE1 concentrations exceeding 10–8 M or with PGF2α in the 10–10 - 10–8 M concentration range.
In agreement with previous data [21-26] such specificity and extremely high sensitivity (less than 1 PGE1 molecule per cell is already sensed) suggest the involvement of receptor-transmitter systems in the PGE1-induced structural reorganization of red cell phospholipids. The present study shows that this structural reorganization proceeds quite differently in ghosts from patients with schizophrenia and healthy subjects (Figure 2).
Figure 2: Influence of prostaglandin E1 on the quenching of tryptophan emission at 330 nm caused by the presence of the sphingomyelin probe ASM in red cell membranes from 12 schizophrenia patients with severe negative symptoms (NS scores > 40) and 10 normal control subjects. Excitation wavelength is 295 nm. Filled circles - patient samples; empty circles – normal controls.
Acknowledgements
We would like to dedicate this paper to the memory of Lev D. Bergelson, DSc, the prime mover of this study, who unfortunately passed away before the work was published. We are deeply indebted to Dr. Julian G. Molotkovsky (Shemyakin Institute of Bioorganic Chemistry, Moscow) and to Dr. Walter Shaw (Avanti Polar Lipids Inc., Alabaster, AL) for preparing and donation of the fluorescent probes used in this study. Dr. A.M. Ponizovsky was supported in part by the Ministry of Immigrant Absorption.References
- Kay SR (1991) Positive and negative yndromes in Schizophrenia. Assessment and Research. New York, NY: Brunner/Mazel, Inc.
- Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, et al. (1999) Stability of the diagnosis of deficit syndrome in schizophrenia. Am J Psychiatry 156: 637-639.
- Kelley ME, van Kammen DP, Allen DN (1999) Empirical validation of primary negative symptoms: independence from effects of medication and psychosis. Am J Psychiatry 156: 406-411.
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr (2001) A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry 58: 165-171.
- Glen AI, Glen EM, Horrobin DF, Vaddadi KS, Spellman M, et al. (1994) A red cell membrane abnormality in a subgroup of schizophrenic patients: evidence for two diseases. Schizophr Res 12: 53-61.
- Horrobin DF, Glen AI, Vaddadi K (1994) The membrane hypothesis of schizophrenia. Schizophr Res 13: 195-207.
- Smythies JR, Alarcon RD, Morere D, Monti JA, Steele M, et al. (1986) Abnormalities of one-carbon metabolism in psychiatric disorders: study of methionine adenosyltransferase kinetics and lipid composition of erythrocyte membranes. Biol Psychiatry 21: 1391-1398.
- Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, et al. (2001) Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry 49: 510-522.
- Ponizovsky AM, Modai I, Nechamkin Y, Barshtein G, Ritsner MS, et al. (2001) Phospholipid patterns of erythrocytes in schizophrenia: relationships to symptomatology. Schizophr Res 52: 121-126.
- Barshtein G, Ponizovsky AM, Nechamkin Y, Ritsner M, Yedgar S, et al. (2004) Aggregability of red blood cells of schizophrenia patients with negative syndrome is selectively enhanced. Schizophr Bull 30: 913-922.
- Ryazantseva NV, Novitskii VV, Kublinskaya MM (2002) Changes in the lipid phase of erythrocyte membranes in patients with paranoid schizophrenia. Bull Exp Biol Med 133: 84-86.
- Haselhorst U, Schenk H, Beyer I, Uebelhack R, Franke E, et al. (1988) Abnormality of gangliosides in erythrocyte membranes of schizophrenic patients. Clin Physiol Biochem 6: 281-284.
- Yao JK, van Kammen DP (1994) Red blood cell membrane dynamics in schizophrenia. I. Membrane fluidity. Schizophr Res 11: 209-216.
- Bonarska-Kujawa D, Pruchnik H, Cyboran S, Żyłka R, Oszmiański J, et al. (2014) Biophysical mechanism of the protective effect of blue honeysuckle (Loniceracaerulea L. var. kamtschatica Sevast.) polyphenols extracts against lipid peroxidation of erythrocyte and lipid membranes. J Membr Biol 247: 611-625.
- Rodgers W, Glaser M (1993) Distributions of proteins and lipids in the erythrocyte membrane. Biochemistry 32: 12591-12598.
- Bonarska-Kujawa D, Kleszczyńska H, Przestalski S (2012) The location of organotins within the erythrocyte membrane in relation to their toxicity. Ecotoxicol Environ Saf 78: 232-238.
- Sonmez M, Ince HY, Yalcin O, Ajdžanović V, Spasojević I, et al. (2013) The effect of alcohols on red blood cell mechanical properties and membrane fluidity depends on their molecular size. PLoS One 8: e76579.
- Moore DJ, Gioioso S, Sills RH, Mendelsohn R (1999) Some relationships between membrane phospholipid domains, conformational order, and cell shape in intact human erythrocytes. Biochim Biophys Acta 1415: 342-348.
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387: 569-572.
- van Kammen DP, Yao JK, Goetz K (1989) Polyunsaturated fatty acids, prostaglandins, and schizophrenia. Ann N Y Acad Sci 559: 411-423.
- Rasmussen H, Lake W (1977) Prostaglandins and the mammalian erythrocyte. In: Prostaglandins in Hematology (Silver, M.J., Smith, J.B., Kcsis, J.J., eds.) Spectrum Publ., New York, p. 132-145.
- Harris RH, Ramwell PW, Gilmer PJ (1979) Cellular mechanisms of prostaglandin action. Annu Rev Physiol 41: 653-668.
- Kury PG, McConnell M (1975) Regulation of membrane flexibility in human erythrocytes. Biochemistry 14: 2798-2803.
- Kury PG, Ramwell PW, McConnell HM (1974) The effect of prostaglandins E1 and E2 on the human erythrocyte as monitored by spin labels. Biochem Biophys Res Commun 56: 478-483.
- Langer R, Rossmanith K, Henrich H (1995) Hemorheological actions of the Ei, E2, Fia, F2a and isoprostane. Clin Hemorheol 15: 829-839.
- McLawhon RW, Marikovsky Y, Thomas NJ, Weinstein RS (1987) Ethanol-induced alterations in human erythrocyte shape and surface properties: modulatory role of prostaglandin E1. J Membr Biol 99: 73-78.
- First MB, Spitzer RL, Gibbon M, Williams JBW (1995) Structured clinical interview for DSM-IV axis I disorders, Patient Edition (SCID-P), version 2. New York State Psychiatric Institute, Biometrics Research.
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261-276.
- Yao JK, Leonard S, Reddy RD (2000) Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res 42: 7-17.
- Molotkovky JG, Nikulina LF, Bergelson LD (1980) Spin and fluorescent labeled lecithins, in: Lipid Biochemical Preparations (LD Bergelson, ed.) Elsevier/North Holland Biochemical Press, Amsterdam- New York-Oxford, pp. 151-154.
- Molotkovsky JG, Dmitriev PI, Molotkovskaya IM, Bergelson LD, Manevich YM (1981) Bioorgan Khim (Russ) 7: 586-600.
- Barshtein G, Bergelson L, Dagan A, Gratton E, Yedgar S (1997) Membrane lipid order of human red blood cells is altered by physiological levels of hydrostatic pressure. Am J Physiol 272: H538-H543.
- Parasassi T, De Stasio G, Ravagnan G, Rusch RM, Gratton E (1991) Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J 60: 179-189.
- Parasassi T, Loiero M, Raimondi M, Ravagnan G, Gratton E (1993) Absence of lipid gel-phase domains in seven mammalian cell lines and in four primary cell types. Biochim Biophys Acta 1153: 143-154.
- Parasassi T, Gratton E (1995) Membrane lipid domains and dynamics as detected by Laurdan fluorescence. J Fluoresc 5: 59-69.
- Johansson LBA, Molotkovsky JG, Bergelson LD (1990) Fluorescence properties of anthrylvinyl lipid probes. Chem Phys Lipids 53: 185-189.
- Bergelson LD, Molotkovsky JG, Manevich YM (1985) Lipid-specific fluorescent probes in studies of biological membranes. Chem Phys Lipids 37: 165-195.
- Keshavan MS, Mallinger AG, Pettegrew JW, Dippold C (1993) Erythrocyte membrane phospholipids in psychotic patients. Psychiatry Res 49: 89-95.
- Parasassi T, Di Stefano M, Loiero M, Ravagnan G, Gratton E (1994) Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys J 66: 120-132.
- Bergelson LD (1995) Dynamic lipid heterogeneity and receptor events. Mol Membr Biol 12: 125-129.
- Molotkovsky JG, Manevich YM, Gerasimova EN, Molotkovskaya IM, PolesskyVA, et al. (1982) Differential study of phosphatidylcholine and sphingomyelin in human high-density lipoproteins with lipid-specific fluorescent probes. Eur J Biochem 122: 573-579.
- Dutta-Roy AK, Sinha AK (1985) Binding of prostaglandin E1 to human erythrocyte membrane. Biochim Biophys Acta 812: 671-678.
- Dutta-Roy AK, Kahn NN, Sinha AK (1991) Interaction of receptors for prostaglandin E1/prostacyclin and insulin in human erythrocytes and platelets. Life Sci 49: 1129-1139.
- Dutta-Roy AK, Hoque L, Paterson BJ (1993) Prostaglandin-E1-binding sites in rabbit erythrocyte membranes. Eur J Biochem 213: 1167-1173.
- Feldberg W (1976) Possible association of schizophrenia with a disturbance in prostaglandin metabolism: a physiological hypothesis. Psychol Med 6: 359-369.
- Abdulla YH, Hamadah K (1975) Effect of ADP on PGE1 formation in blood platelets from patients with depression, mania and schizophrenia. Br J Psychiatry 127: 591-595.
- Kim SW, Schäfer MR, Klier CM, Berk M, Rice S, et al. (2014) Relationship between membrane fatty acids and cognitive symptoms and information processing in individuals at ultra-high risk for psychosis. Schizophr Res158: 39-44.
- Nuss P, Tessier C, Ferreri F, De Hert M, Peuskens J, et al. (2009) Abnormal transbilayer distribution of phospholipids in red blood cell membranes in schizophrenia. Psychiatry Res 169: 91-96.
- Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA (1994) Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 60: 189-194.
- Ponizovsky AM, Barshtein G, Bergelson LD (2003) Biochemical alterations of erythrocytes as an indicator of mental disorders: an overview. Harv Rev Psychiatry 11: 317-332.
- Fritze J, Koronakis P, Riederer P (1988) Erythrocyte membrane proteins in psychiatric disorders and controls. J Affect Disord 15: 187-190.
- Dietrich-Muszalska A, Kontek B (2010) Lipid peroxidation in patients with schizophrenia. Psychiatry Clin Neurosci 64: 469-475.
- Nuss P, Tessier C, Ferreri F, De Hert M, Peuskens J, et al. (2009) Abnormal transbilayer distribution of phospholipids in red blood cell membranes in schizophrenia. Psychiatry Res 169: 91-96.