Journal of Neurology and Psychology
Download PDF
Research Article
*Address for Correspondence: Shirley Telles, Ph.D., Director, Patanjali Research Foundation, Patanjali Yogpeeth, Haridwar, Uttarakhand 249405, India, Tel: +91.01334.244805; Fax: +91.01334.244805; E-mail: shirleytelles@gmail.com
Citation: Deepeshwar S, Telles S. Auditory Information Processing During Meditation Based on Evoked Potential Studies. J Neurol Psychol. 2013;1(2): 7.
Copyright © 2013 Singh D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 1, Issue: 2
Submission: 01 October 2013 | Accepted: 16 December 2013 | Published: 20 December 2013
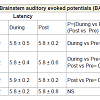
The BAEP recordings showed that the peak latency of a specific component, wave V (5.8 – 6.0 ms), increased significantly during dharana, ekagrata, and cancalata sessions, but there was no change during the practice of dhyana [13]. Since wave V is considered to correspond to the inferior colliculus located in the tectum (midbrain) [10,12], this suggested that neural transmission at the level of midbrain may be improved by meditation without focusing. The results also suggested that dhyana practice alone does not delay auditory sensory transmission at the brainstem level, whereas dharana practice is associated with a delay which was also seen in the practices of ekagrata and cancalata. The traces of BAEPs before and after meditation are given in Figure 1a and 1b respectively.
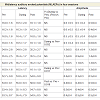
The MLAEPs show the prolonged peak latencies of two components (the Na wave and the Pa wave) during meditation. The Pa wave amplitude decreased during all four states. Prolonged latencies of the Na and Pa wave suggest delayed auditory information transmission at mesencephalic – diencephalic levels and at the level of the primary auditory cortex (i.e., the neural generators corresponding to the Na and Pa waves) [18,19]. The traces of MLAEPs before and after meditation are given in Figure 2 and 2b respectively.
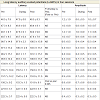
There were decreased peaks amplitudes of the P1 and P2 waves after random thinking and non-meditative focusing and the N2 wave after non-meditative focusing suggesting that the neural activity was reduced at the level of secondary auditory cortex, auditory association complex and anterior cingulate cortex, respectively [26]. The reason for decrease in P1, P2 and N2 amplitudes may be due to selective inhibition of certain areas within the primary, auditory association complex and secondary auditory cortex suppressing sensory responses to reduce distracting auditory stimuli, which could prevent the participants directing their attention on instructions [27] during random thinking and non-meditative focusing. The traces of MLAEPs before and after meditation are given in Figure 3 and 3b respectively.
Auditory Information Processing During Meditation Based on Evoked Potential Studies
Deepeshwar Singh1 and Shirley Telles1,2*
- 1Indian Council of Medical Research Center for Advanced Research in Yoga and Neurophysiology, Swami Vivekananda Yoga Anusandhana Samsthana, Bengaluru, India
- 2Patanjali Research Foundation, Haridwar, Uttarakhand, India
*Address for Correspondence: Shirley Telles, Ph.D., Director, Patanjali Research Foundation, Patanjali Yogpeeth, Haridwar, Uttarakhand 249405, India, Tel: +91.01334.244805; Fax: +91.01334.244805; E-mail: shirleytelles@gmail.com
Citation: Deepeshwar S, Telles S. Auditory Information Processing During Meditation Based on Evoked Potential Studies. J Neurol Psychol. 2013;1(2): 7.
Copyright © 2013 Singh D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 1, Issue: 2
Submission: 01 October 2013 | Accepted: 16 December 2013 | Published: 20 December 2013
Abstract
Background: Auditory evoked potentials (AEPs) were recorded to examine the neurophysiological correlates of four mental states described in ancient yoga texts. These are (i) focused attention (dharana), (ii) contemplation (dhyana) (iii) random thinking (cancalata) and (iv) non meditative focused thinking (ekagrata). The auditory evoked potentials allowed changes from the periphery (cochlear nucleus) to the center (auditory association cortex) were measured.Method: There were sixty male participants with ages ranging from 18 to 45 years (group mean age ± SD, 27.0 ± 8.3 years) who were assessed in four sessions. These four sessions were i) random thinking (cancalata), ii) non meditative focusing (ekagrata), (iii) meditative focusing (dharana), and (iv) contemplation (dhyana). The order of the sessions was randomly assigned.The data were analysed with repeated measure ANOVA followed by a post hoc analysis.
Results: The BAEPs results showed that the wave V peak latency significantly increased in random thinking (p<0.05), non-meditative focused thinking (p<0.01) and meditative focused thinking (p<0.05) sessions which suggest that during meditation there was no change in processing time of information at the inferior colliculus. MLAEPs results showed that there were significantly increased latencies of the Na and Pa waves during meditation (p<0.05) which suggest reduced auditory information transmission at the medial geniculate and primary auditory cortices. The LLAEPs result showed that there was a significant decrease in the amplitude of P1, P2 and N2 waves during random thinking (p<0.01; p<0.001; p<0.01, respectively) and non-meditative focused thinking (p<0.01; p<0.01; p<0.05, respectively) sessions and a decrease in the latency of P2 wave during and after meditation (p<0.001) session which suggest facilitated auditory transmission at the auditory association cortex. The changes in P300 event related potentials suggested that meditation improved the interaction between the frontal lobe; hippocampus and temporal-parietal parts of the brain during the P300 auditory oddball task. Hence, through brainstem, midlatency, long latency and event related potentials changes in the auditory sensory pathway were assessed in different mental states.
Conclusion: Meditation showed no changes in auditory information transmission at the collicular level, but decreases it at the geniculate, primary and association auditory cortices.
Background
Meditation has been described as a mental training through which practitioners try to develop and increase flexibility and awareness of their mental processes, culminating in mental stability [1]. Practice of meditation over a period of time produces definite changes in perception, attention, and cognition [2]. Meditation is recognized as a specific consciousness state in which deep relaxation and increased internalized attention exist at the same time [3].The concepts of meditation described in ancient yoga texts are associated with heightened attention or even of being aware of the experience as it happens. In Patanjali’s Yoga Sutras (circa 900 B.C.) two meditative states are described [4]. The first is focusing with effort (or dharana) to confine the mind within a limited mental area (Patanjali’s Yoga Sutras, Chapter 3, Verse 1). The next stage is effortless expansion or dhyana (Patanjali’s Yoga Sutras, Chapter 3, Verse 2), which is the uninterrupted flow of the mind towards the object chosen for meditation. The practice of dharana is supposed to precede the practice of dhyana. When the mind is not in meditation, another ancient yoga text says that it may be in two other states, cancalata which is a state of random thinking (Bhagavad Gita, circa 500 B.C. Chapter 6, Verse 34) and ekagrata (Bhagavad Gita, Chapter 6, Verse 12), or focused attention without meditation, during which the attention is directed to a number of associated thoughts.
These four mental states have been studied to evaluate auditory information processing from the cochlear nerve at the periphery to the association cortices located centrally. Auditory evoked potentials were chosen to begin with, instead of other modalities of evoked potentials to avoid compounding with any other sensory or motor potentials. The auditory modality of stimuli was particularly chosen as it was found to be least disturbing to the meditator during their practice [5]. It is the premise that conscious processes actively involve several cortical mechanisms and also that corticofugal control processes may exert significant alterations in the processing of information at brainstem, thalamic and cortical levels [6-9]. Evoked potentials which form the basis of this report include brainstem (0-10 ms), mid latency (10-100 ms), long latency auditory evoked potentials (100-250 ms) and the P300 event-related evoked potentials recorded with the auditory oddball paradigm (280-450 ms). For each auditory evoked potential component the peak latency and peak amplitude has been assessed. The peak latency (msec) is defined as the time from stimulus onset to the point of maximum positive or negative amplitude within a specified latency window. The peak amplitude (μV) is defined as the voltage difference between a pre-stimulus baseline and the largest positive and negative going peak within a latency window. A decrease in peak latency is considered as suggestive of facilitated transmission due to increased speed of conduction in the underlying neural generators [10]. Conversely, an increase in peak latency can be assumed to suggest inhibited transmission due to slower conduction in the underlying neural generators. With respect to changes in peak amplitude, an increase in the amplitude of an evoked potential component has been interpreted as being indicative of effective activation of the underlying neural generator, with recruitment of additional neurons [11].
A series of experiments on auditory evoked potentials were carried out between June 2007 and December 2012 to understand the neurophysiological effects of two meditative states (dharana and dhyana) and two non-meditative states (cancalata and ekagrata).
Method
Sixty healthy male volunteers whose ages ranged between 20 and 45 years (group mean age ± SD, 27.0 ± 8.3 years) were recruited for recording of BAEPs, MLAEPs, LLAEPs and P300 ERPs. All of them were residing at a yoga center in South India and were actively engaged in practicing yoga. Their health status was based on a routine case history and clinical examination. All the participants had a minimum of 6 months experience of meditation (group average experience ± SD, 22.5 ± 17.5 months) on the Sanskrit syllable, OM. This meditation technique can be separately practiced as dharana (focusing on thoughts of OM) and dhyana (effortless focusing on OM). Participants were trained to practice the two techniques (dharana and dhyana) separately and at will. To attempt to ensure that all of them were doing it correctly, they were given a 3-month orientation course, during which time they were supervised by an experienced meditation teacher.Assessments
The assessments included (i) brainstem auditory evoked potentials, (ii) mid latency auditory evoked potentials (iii) long latency auditory evoked potentials and (iv) P300 auditory event related potentials with the auditory oddball paradigm. Each of these assessments and the results obtained will be discussed below in detail.
Statistical analysis
Statistical analysis was done using SPSS (Version 16.0). Data were tested for normality by Kolmogorov-Smirnov test. Since the same individuals were assessed in repeated sessions on separate days (i.e., random thinking, non-meditative focused thinking, meditative focusing and meditation), repeated measures analysis of variance was used (ANOVA). Repeated measures analyses of variance (ANOVA) were performed with two ‘within subjects’ factors, i.e., Factor 1: Sessions; Random thinking, Non-meditative focused thinking, Meditative focusing and Meditation, and Factor 2: States; Before, During (Dur1 to Dur4), and After. Repeated measures ANOVAs were carried out for each component of BAEPs, MLAEPs, LLAEPs and P300 ERPs separately, for both peak latencies and peak amplitudes. This was followed by a post-hoc analysis with Bonferroni adjustment for multiple comparisons between the mean values of different states (“During” and “After”). All comparisons were made with the respective “Before” state.
Results
The group mean values ± S.D. for the peak latencies (ms) and peak amplitudes (μV) for each component of BAEPs, MLAEPs and LLAEPs in four sessions (random thinking, non-meditative focused thinking, meditative focusing and meditation) in Before, During and After states are given in Table 1, Table 2 and Table 3, respectively.Discussions
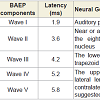
The results of the BAEPs, MLAEPs, LLAEPs and P300 ERPs are discussed below.Brain stem auditory evoked potentials (BAEPs)Brainstem auditory evoked potentials (BAEPs) provide an objective physiological index of auditory function at a subcortical level [12]. They reflect neuronal activity in the cochlear nerve, cochlear nucleus, superior olive and inferior colliculus of the brainstem. BAEPs (0 – 10 ms) were recorded using standard methods [13]. The peak latency and peak amplitude of BAEP components were measured. The neural generators of these components are given in Table 4. A typical trace is shown in Figure 1.The BAEP recordings showed that the peak latency of a specific component, wave V (5.8 – 6.0 ms), increased significantly during dharana, ekagrata, and cancalata sessions, but there was no change during the practice of dhyana [13]. Since wave V is considered to correspond to the inferior colliculus located in the tectum (midbrain) [10,12], this suggested that neural transmission at the level of midbrain may be improved by meditation without focusing. The results also suggested that dhyana practice alone does not delay auditory sensory transmission at the brainstem level, whereas dharana practice is associated with a delay which was also seen in the practices of ekagrata and cancalata. The traces of BAEPs before and after meditation are given in Figure 1a and 1b respectively.
Midlatency auditory evoked potentials (MLAEPs)
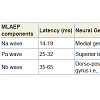
Midlatency auditory evoked potentials (MLAEPs) have been used to assess subcortical and cortical changes in meditation [14]. It is believed that even if the main changes occur in the cortex, corticoefferent connections would result in sub-cortical changes [11]. The mid latency auditory evoked potentials reflect neural activity at the mesencephalic or diencephalic level [15], the superior temporal gyrus [16], and the dorso-posterior-medial part of the Heschl’s gyrus, i.e., the primary auditory cortex [17]. The peak latency and peak amplitude of MLAEPs were measured with three components which correspond to the different neural generators given in Table 5. A typical trace is shown in Figure 2. MLAEPs (10 – 100 ms) were recorded using standard methods [18].
The MLAEPs show the prolonged peak latencies of two components (the Na wave and the Pa wave) during meditation. The Pa wave amplitude decreased during all four states. Prolonged latencies of the Na and Pa wave suggest delayed auditory information transmission at mesencephalic – diencephalic levels and at the level of the primary auditory cortex (i.e., the neural generators corresponding to the Na and Pa waves) [18,19]. The traces of MLAEPs before and after meditation are given in Figure 2 and 2b respectively.
Long latency auditory evoked potentials (LLAEPs)
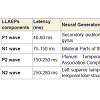
Long latency auditory evoked potentials (LLAEP) assess auditory information processing at the central level. LLAEPs measures are thought to reflect the activation of primary auditory cortex and association cortices [20,21]. In long latency auditory evoked potentials, currently the neural generators is believed to be due to activity at the secondary auditory cortex in the lateral Heschl’s gyrus [17], bilateral parts of the auditory cortex (superior temporal gyrus) [22], and auditory association complex [20] which responds to input from all sensory modalities [22] and left superior temporal gyrus and bilateral medial temporal lobe structure [23]. The peak latency and peak amplitude of LLAEP components (100 – 300 ms) were measured [24,25]. The neural generators of these components are given in Table 6. A typical trace is shown in Figure 3.
There were decreased peaks amplitudes of the P1 and P2 waves after random thinking and non-meditative focusing and the N2 wave after non-meditative focusing suggesting that the neural activity was reduced at the level of secondary auditory cortex, auditory association complex and anterior cingulate cortex, respectively [26]. The reason for decrease in P1, P2 and N2 amplitudes may be due to selective inhibition of certain areas within the primary, auditory association complex and secondary auditory cortex suppressing sensory responses to reduce distracting auditory stimuli, which could prevent the participants directing their attention on instructions [27] during random thinking and non-meditative focusing. The traces of MLAEPs before and after meditation are given in Figure 3 and 3b respectively.
P300 auditory oddball paradigm
The P300 component of event-related potentials (ERPs) is considered a cognitive neuro-electric phenomenon because it is generated in psychological tasks when subjects attend to and discriminate between stimuli that differ from one another in some dimension [28]. It is also called the “oddball” paradigm since subjects are required to distinguish between frequent and rare stimuli presented as a random series; responding to the rare (target) stimulus and ignoring the frequent stimuli. The generation of a P300 positive deflection is believed occur from the interaction between the frontal lobe and hippocampal and temporoparietal function [29]. The primary neural generator for the P300 components are in the anterior cingulate and hippocampal formation [30].
There was a significant reduction of the P300 peak amplitude after random thinking session (cancalata) whereas the peak amplitude significantly increased after focused meditation (dharana) and meditation without focusing (dhyana) [31]. These results show that following meditation with focusing and meditation without focusing, the ability to perform the P300 auditory oddball task was better, while after a session of equal duration of random thinking reduced. The neuro-electric events which underlie the P300 arise from the interaction between the frontal lobe; hippocampus and temporo-parietal function parts of the brain known to be involved in meditation [28] (Figure 4).
Summary
Auditory evoked potentials, a noninvasive method of evaluating auditory information transmission from the periphery to the center. Brainstem, mid latency, long latency, and P300 auditory event related potentials were recorded in meditation, meditative focusing, random thinking and non-meditative focused thinking. The findings demonstrated that meditation had distinctly different effects compared to the other three states.In summary during meditation there was:
i) A decrease in the brainsteim auditory evoked potentials at wave V peak latency suggesting reduces the speed of transmission in the midbrain (inferior colliculous).
ii) Peak latencies of midlatency of Na and Pa wave were reduced suggesting reduction in speed of transmission at mesencephalic – diencephalic region and Heschle’s gyrus.
iii) The peak amplitude of the P2 component of LLAEPs, evoke potentials was increase suggesting involvement of large area within the auditory association cortex along with recruitment of more neurons.
iv) P300 amplitude of auditory event related potentials increased while the latency reduced suggesting improved attention for the auditory oddball.
Hence, meditation is distinct state in which attention to auditory stimuli improve while the speed of auditory information up to the primary appears to be slower.
Acknowledgements
The authors gratefully acknowledge the funding from the Indian Council of Medical Research (ICMR), Government of India, as part of a grant (Project No. 2001-05010) towards the Center for Advanced Research in Yoga and Neurophysiology (CAR-Y&N). The authors would like to thank all ICMR-CAR scientists, Naveen K.V., Manjunath N.K., Subramanya P., Sanjay K, Raghvendra B.R. for their help at different stages of this work.References
- Wallace BA, Shapiro SL (2006) Mental balance and well-being: building bridges between Buddhism and Western psychology. Am Psychol 61: 690-701.
- Brown DP (1977) A model for the levels of concentrative meditation. Int J Clin Exp Hypn 25: 236-273.
- Murata T, Takahashi T, Hamada T, Omori M, Kosaka H, et al. (2004) Individual trait anxiety levels characterizing the properties of zen meditation. Neuropsychobiology 50: 189-194.
- Taimni IK (1994) The Science of Yoga: The Yoga-sutras of Patanjali in Sanskrit with Transliteration in Roman, Translation and Commentary in English. Theosophical Publishing House.
- Telles S, Joseph C, Venkatesh S, Desiraju T (1993) Alterations of auditory middle latency evoked potentials during yogic consciously regulated breathing and attentive state of mind. Int J Psychophysiol 14: 189-198.
- Desiraju T (1979) Electrophysiology of Prefrontal and Dorsolateral Cortex elucidating the basis and nature of Higher nervous associations in primates. In: (Ed.) MABB, editor. Brain Mech. Mem. Learn. From single neuron to man, New York, p. 79-89.
- Desiraju T (1984) Neurophysiology and Consciousness. An integrated non - dualist evolutionary theory. In: (Eds.) DG and PIK, editor. Front. Physiol. Res., Academy of Science, Canberra: Australian Academy of Science, Canberra, and Cambridge University Press, p. 325-333.
- Pribram KH, McGuinness D (1992) Attention and para-attentional processing. Event-related brain potentials as tests of a model. Ann N Y Acad Sci 658: 65-92.
- Brazier MAB (1979) Electrophysiology of Prefrontal and Dorsolateral Cortex elucidating the basis and nature of Higher nervous associations. Brain Mech. Mem. Learn. from single neuron to man, Raven Press, Limited, p. 400.
- Malhotra A (1997) Auditory evoked responses in clinical practice. Springer-Verlag.
- Woods DL, Clayworth CC (1985) Click spatial position influences middle latency auditory evoked potentials (MAEPs) in humans. Electroencephalogr Clin Neurophysiol 60: 122-129.
- McEvoy TM, Frumkin LR, Harkins SW (1980) Effects of meditation on brainstem auditory evoked potentials. Int J Neurosci 10: 165-170.
- Kumar S, Nagendra H, Naveen K, Manjunath N, Telles S (2010) Brainstem auditory-evoked potentials in two meditative mental states. Int J Yoga 3: 37-41.
- Telles S, Nagarathna R, Nagendra HR, Desiraju T (1994) Alterations in auditory middle latency evoked potentials during meditation on a meaningful symbol--“Om”. Int J Neurosci 76: 87-93.
- Deiber MP, Ibanez V, Fischer C, Perrin F, Mauguiere F (1988) Sequential mapping favours the hypothesis of distinct generators for Na and Pa middle latency auditory evoked potentials. Electroencephalogr Clin Neurophysiol 71: 187-197.
- Kileny P, Paccioretti D, Wilson AF (1987) Effects of cortical lesions on middle-latency auditory evoked responses (MLR). Electroencephalogr Clin Neurophysiol 66: 108-120.
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P (1994) Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol 92: 204-214.
- Telles S, Raghavendra BR, Naveen KV, Manjunath NK, Subramanya P (2012) Mid-latency auditory evoked potentials in 2 meditative states. Clin EEG Neurosci 43: 154-160.
- Subramanya P, Telles S (2009) Changes in midlatency auditory evoked potentials following two yoga-based relaxation techniques. Clin EEG Neurosci 40: 190-195.
- Crowley KE, Colrain IM (2004) A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol 115: 732-144.
- Wolpaw JR, Wood CC (1982) Scalp distribution of human auditory evoked potentials. I. Evaluation of reference electrode sites. Electroencephalogr Clin Neurophysiol 54: 15-24.
- Näätänen R, Picton T (1987) The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology 24: 375-425.
- Halgren E, Baudena P (1992) Endogenous potentials recorded in the human superior temporal plane, including Heschl’s gyms. Present. 10th Int. Congr. Evoked Potentials (EPIC X), Eger, Hungary, p. 55.
- Vaughan HG Jr, Ritter W (1970) The sources of auditory evoked responses recorded from the human scalp. Electroencephalogr Clin Neurophysiol 28: 360-367.
- Picton TW, Hillyard SA (1974) Human auditory evoked potentials. II. Effects of attention. Electroencephalogr Clin Neurophysiol 36: 191-199.
- (2011) ICMR Report 2011. Neurophysiological correlates of phases of wakefulness and sleep in meditators. Bangalore, India (Unpublished data).
- Nunez A, Malmierca E (2007) Corticofugal modulation of sensory information. Adv Anat Embryol Cell Biol 187: 1-74.
- Polich J, Kok A (1995) Cognitive and biological determinants of P300: an integrative review. Biol Psychol 41: 103-146.
- Halgren E, Marinkovic K, Chauvel P (1998) Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol 106: 156-164.
- Polich J (1999) Electroencephalography: Basic principles, Clinical applications and related fields p. 1073-1091.
- (2012) ICMR Report 2012. Neurophysiological correlates of phases of wakefulness and sleep in meditators. Bangalore, India (Unpublished data).