Journal of Nutrition & Health
Download PDF
Research Article
*Address for Correspondence: Hassan IH El-Sayyad, Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt, Tel: 0020502254850; E-mail: elsayyad@mans.edu.eg
Citation: El-Sayyad HIH, Ali DA, Hanafy ME. Role of Fish Oil on Amelioration of Injured Cornea and Lens of Diabetic and Hypercholesterolemic Male Wistar Rats. J Nutri Health. 2016;2(1): 9.
Copyright © 2016 El-Sayyad HIH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Nutrition and Health | ISSN: 2469-4185 | Volume: 2, Issue: 1
Submission: 26 November, 2015 | Accepted: 05 January, 2016 | Published: 12 January, 2016
Scanning electron microscopic observation of lens
Light and TEM observation of cornea
Diabetic and/ or hypercholesterolemia possessed marked damage of the cornea characterized by apparent thinning of the corneal epithelium and damaged epithelium with either vacuolated or pyknotic nuclei. Hyalinized and degenerated Bowman’s layer was detected. The integrity of the stromal architecture was disorganized and infiltrated by numerous necrotic foci. The keratocytes lacked normal ordinary structure and degenerated. Multifocal or diffuse leukocyte inflammatory cells were observed especially in the peripheral stromal layers. In case of diabetic and hypercholesterolemic group, there was a detected increase of neovascularization manifested by numerous vacuolated regions outlined internally by flattened cells suspected to be endothelial cells. The corneal endothelium lining the Descemet’s membrane become thickened (Figures 1C, 1E and 1G).
In experimental diabetic and hypercholesterolemic group, there was a detected increase of epithelial cells with pyknotic nuclei and massive deterioration of stroma with presence of neovascularization and pyknotic keratocytes. The Descemet’s membrane and their endothelial lining layer appeared hyalinized (Figures 4A-4C). Although, fish oil supplementation to experimental diabetic and hypercholesterolemic group, improved the cytological structure, there was a detected necrotic foci in stroma and less improvement in endothelium (Figures 4A-4C1).
Similar findings were reported in cornea of 24 & 30 M old rats [56].
Role of Fish Oil on Amelioration of Injured Cornea and Lens of Diabetic and Hypercholesterolemic Male Wistar Rats
Hassan IH El-Sayyad*, Doaa A. Ali and Mohamed E Hanafy
- Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt
*Address for Correspondence: Hassan IH El-Sayyad, Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt, Tel: 0020502254850; E-mail: elsayyad@mans.edu.eg
Citation: El-Sayyad HIH, Ali DA, Hanafy ME. Role of Fish Oil on Amelioration of Injured Cornea and Lens of Diabetic and Hypercholesterolemic Male Wistar Rats. J Nutri Health. 2016;2(1): 9.
Copyright © 2016 El-Sayyad HIH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Nutrition and Health | ISSN: 2469-4185 | Volume: 2, Issue: 1
Submission: 26 November, 2015 | Accepted: 05 January, 2016 | Published: 12 January, 2016
Abstract
The association between diabetes and hypercholesterolemia and cataract represents the main public health problem. Little information about the biochemical changes in lens during the progress of disease parallel with lens ultrastructure. Also, the interrelationship between lens and cornea and the dramatic effects of both diseases in cornea was still not clearly illustrated. Our objective was designed to the role of diabetes and hypercholesterolemia in the development of lens and corneal damages and the ameliorated role of fish-oil-treatment. Sixtyfour male albino rats of Wistar strain were arranged into eight groups (n=8). These are control, fish oil-supplementation, diabetes (single ip stretozotocin of 40 mg/kg body weight in citrate buffer pH 4.6), diabetes and fish oil, hypercholesterolemia (fed on diet rich of 3% cholesterol), hypercholesterolemia and fish oil, diabetes and hypercholesterolemia, diabetes and hypercholesterolemia and fish oil. Treatments were carried out for 16 weeks. Rats were sacrificed and lenses and cornea were separated. Lenses were examined biochemically for glycated protein, endothellin, adhesion molecules and zinc and iron contents as well as scanning electron microscopy. Cornea was examined at light and transmission electron microscopy.The lens of diabetic and/or hypercholesterolemia exhibited apparent increase of carbonylated proteins and glycation end products, endothelin-1, adhesion molecules (ICAM-1 & VCAM-1), zinc and iron parallel with disorganized ball and socket and degeneration of lens fibers. The cornea of both diabetic and or hypercholesterolemic possessed damage of corneal epithelium, hyalinization and degeneration of both Bowman’s and stromal collagenous layers associated with diffuse leukocytic inflammatory cells in the peripheral collagenous stromal layers and degeneration of endothelium lining the Descemet’s membrane. Hypercholesterolemia showed the least dramatic effect in comparison with diabetes. Fishoil supplementation ameliorated the lens and corneal structure and improved the biochemical changes.
The author finally concluded that the amelioration role of fish oilsupplementation attributed to the reduction of glycated proteins, endothelin-1, adhesion molecules and lens zinc and iron contents which reflected in the structural components of lens fibers as well as improved the elementary structures of cornea.
Keywords
Lens; Cornea; Diabetes; Hypercholesterolemia; Fish oilBackground
Diabetes mellitus is a worldwide disease widespread is in association with obesity; and affected more than 285 million people and expected to increase to 439 million by 2030 [1]. Diabetes and hypercholesterolemia are inter-related with each other associated with the increased of oxidative stress and the development of cataract which represent the main integral part in impairing vision affecting 80 million patients and 18 million blindness [2-6].The cornea is present in front of the lens with characteristic transparencies for light to penetrate and vision of the individual. Diabetes and hypercholesterolemia represent the main cause of disrupted lens metabolism and altering protein configuration causing cataract [3,7-10].
Also, corneal arcus was found to be associated with elevated cholesterol, especially in the young as well as in patients with familial hypercholesterolemia [11,12]. Cholesterol deposition was reported in cornea of rabbits [13]. STZ-diabetic mice possessed a decrease of dendritic cells density in ob/ob mice in comparison with the control [14]. Obese and type I & II diabetic C57Bl/6J mice were found to develop motor and sensory nerve conduction deficits as well as a decrease in sub-epithelial corneal nerves, innervations of the corneal epithelium [15]. Diabetes mellitus was found to impair vision via increasing corneal thickness, inducing corneal edema, and altered epithelial basement membrane and reducing anterior stromal keratocyte, endothelial cell densities and altered intensities of corneal innervations [16-18].
Diabetic-related cataract is contributed mainly to impairment of vision [19]. Diabetic lens possessed abnormal curved lenses with increased lens thickness and decreased lens equivalent refractive index and reduction of lens equatorial diameter [20]. Diabetes was found to increase cell death via disturbed interaction of aBcrystallin by Bax and caspases [21]. In experimental rat models of types 1 and 2 diabetes, the levels of α-, ß- and αA-crystallins (αAC) and γ-crystallins were markedly decrease meanwhile γ-crystallin levels were increased coincides with a depletion of both reduced glutathione and adenosine triphosphate [22,23]. On the other hand, Obesed rat possessed increased sorbitol level of their lenses [24]. Increased level of cholesterol oxide was detected in patient with cataractous lenses [25]. Also, Girao et al. confirmed these findings by exposure transparent membranes from clear lenses to free radicals generato 2-amidinopropane and observed the presence of 7 alphahydroxycholesterol (6%), 7 beta-hydroxycholesterol (19%), 5 & 6 alpha-epoxycholestanol (1%) and 7-ketocholesterol (74%) [25]. X-ray diffraction of cataractous human lenses revealed increased level of cholesterol domains [26]. El-Sayyad et al. reported the presence of cataractous lenses in offspring maternally fed on high cholesterol diet [6].
Fish oil composed of two main inclusions, omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the precursors of eicosanoids (Moghadasian, 2008). In vitro studies carried out by Perales et al. revealed that 25-hydroxycholesterol led to apoptic cell death of corneal smooth muscle cells, initiated by increased level of Bax protein and overexpression of Bax gene. Fish oil-treatment reversed these dramatic changes to the anti-apoptotic pathways [27].
Phytosterol esters was found to have a role in countering hypercholesterolemia-related changes in the brain by decreasing the cholesterol levels, increasing the phospholipid levels and increasing the level of antioxidant enzymes [28]. Fish oil was found to inhibit the production of proinflammatory mediators in rats fed on hypercholesterolemic diet [29].
The present study aims to evaluate the role of Menhaden fish oil in ameliorating the dramatic changes in cornea and lens of diabetic and hypercholesterolemic rats.
Materials and Methods
ChemicalsAll of the chemical used were of highest purity. Streptozotocin (N-(Methylnitrosocarbamoyl)-a-D-glucosamine), cholesterol (3ß-Hydroxy-5-cholestene, 5-Cholesten-3ß-ol) and Menhaden fish oil were supplied from Sigma-aldrich company (USA).
Experimental work
Sixty-four male albino rats weighing approximately 100 g body weight, obtained from Breading Farm, Ministry of Health, Giza, Egypt. They were fed on standard diet and water was allowed ad libitum throughout the experimental period. The animals were housed in good ventilation with 12 hour light and dark cycle. Male rats were fed on diet containing 3% cholesterol for 16 weeks. Diabetes was carried out and animals allowed feeding on normal healthy diet for the mentioned period treatment. Rats were arranged into eight groups (n=8) such as Control (C), Fish oil-treatment (F) (orally administered every other day at dose 100 mg/kg body weight), hypercholesterolemic-group (H), hypercholesterolemic & fish oiltreatment (HF), diabetic-group (D), diabetic and fish oil-treatment (DF), combined hypercholesterolemic and diabetic group (HD) and combined hypercholesterolemic and diabetic group and fish-oiltreatment (HDF). The animals were maintained on diabetes and / or hypercholesterolemia as well as treatment with fish oil for 16 weeks.
Induction of diabetes
Experimental diabetes mellitus was induced by a single interperitoneal injection of streptozotocin (40 mg/kg) in citrate buffer (0.05 M) (pH 4.5) [30]. Treatment was carried out for 16 weeks. Control animals were treated with physiological saline as vehicle. Hyperglycemia was verified by measuring the blood glucose within the range of 180-220 mg/dL.
Induction of hypercholesterolemia
The experimental group was fed a hypercholesterolemic diet composed of 3% cholesterol and 15% cocoa butter and 0.2% cholic acid and 0.2% thiouracil for 16 wks [31]. The control group was fed on a normal healthy diet free from atherogenic components.
At the end of experiment, male rats of the control and treated groups were sacrificed and ocular regions were removed and dissected for separation cornea and lens and investigated as follows:
Biochemical investigation: (a) Carbonyl & glycation end products: Protein carbonylation was assessed by the reaction of carbonyl groups with 2, 4-dinitrophenylhydrazine (DNPH) to form 2, 4-dinitrophenylhydrazones which determined spectrophotometrically at 360-385 nm against standard carbonyl protein. AGE products contain CML, pentosidines and other AGE structures which is determined using ELISA Kit of Cell Biolabs, (Inc., 7758 Arjons Drive, San Diego, CA 92126 USA, Cat.No. STA-317). AGE content in protein samples was determined by comparing its absorbance with that of a known advanced glycation end product and bovine serum albumin (AGE-BSA) standard curve.
(b) Endothelin: Endothelin-1(ET-1) was determined using Endothelin-1 of R&D system (Inc. 614 McKinley Place NE Minneapolis, MN 55413, Cat.No. DET100). The method is based on competitive inhibition reaction between biotin labelled Endothelin1 and unlabelled ET-1 with the pre-coated antibody specific to ET- 1. Avidin conjugated with horseradish peroxidase is added and the amount of bounded HRP is proportional to the amount of ET-1 in the lens which is measured by spectrophotometer at 450 nm (within 30 min to avoid fading). Standard curve was plotted using ET-1.
(c) Determination of adhesion molecules (ICAM-1 and VCAM-1): Adhesion molecules were determined by ELISA kit of R&D Systems, Inc (614 McKinley Place NE Minneapolis, MN 55413, USA, Cat. No. DET100). Color development was carried out and the optical density was determined at a wavelength of 450 nm with the wavelength corrected at 620 nm.
(d) Determination of zinc and iron contents: Lens samples of both control and experimental groups, washed thoroughly with distilled water and weighed. They were dried and mixed well by using chloroform methyl mixture 2:1 for lipid extraction. A known weighed of sample per each experimental group was digested by 1 ml of nitric acid at highest purity and diluted with 4 ml bi-distilled water. Zinc and iron were measured by atomic absorption spectrometry [32].
(e) Scanning electron microscopic investigation: Lenses of both control and treated groups were fixed in 2.5% glutaraldhyde (pH 7.4) and dehydrated in ascending grades of ethyl alcohol. The specimens were dried in a carbon dioxide critical point apparatus, mounted in stubs and coated with a thin film of gold at DC sputtering and investigated under scanning electron microscope JOEL5300 JSM (Musashino 3-chome akishima Tokyo 196-8558, Japan).
(f) Histological investigation: Cornea of both control and treated groups and fixed in 10% phosphate buffered formalin (pH 7.4). They were dehydrated in ascending grades of ethyl alcohol, cleared in xylol and mounted in molten paraplast at 58-62 ºC. Five micron sections were obtained stained with hematoxylin & eosin and assessed histopathologica changes under a bright field Olympus microscope.
(g) Transmission electron microscopy: Cornea of the studied animal groups were removed and fixed in 2% buffered glutaraldhyde, dehydrated in ascending grades of ethyl alcohol, cleared in propylene oxide and mounted in epoxy resin. Ultrathin-sections were cut and stained with lead citrate and uranyl acetate and examined in Joel transmission electron microscopy 100X (Tokyo 196-8558, Japan).
(h) Statistical analysis: Data were presented as mean±standard error. The statistical analysis was performed with multi-variant analysis of variance (MANOVA) using SPSS (version 13) software package for windows for comparing the multivariations between each specie and p< 0.05 was considered statistically significant.
Results
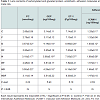
Biochemical assays Table 1 illustrates lens protein carbonylation, glycation end product, endothelin, adhesion molecules (ICAM-1 & VCAM-1) and zinc and iron contents of diabetes and or hypercholesterolemia and amelioration role of fish oil-supplementation. Comparing with the control, there were marked increase of protein carbonylation (PC) and glycation end product (GEP) of diabetes being 3.57±0.32 and 4.14±0.19 respectively. In hypercholesterolemia, their levels were increased but less than diabetes-treatment being 3.19±0.17 and 3.88±0.18 respectively. However, diabetes and hypercholesterolemia exhibited apparent increase being 3.50±0.22 and 4.29±0.10 respectively. The levels endothelin-1 and adhesion molecules (ICAM-1 & VCAM-1) attained marked increase in diabetic and hypercholesterolemia in comparison with either diabetes or hypercholesterolemia-treatment.
Also, the lens zinc and iron contents were markedly increased in diabetic and or hypercholesterolemia. Fish oil-supplementation possessed ameliorations of the assayed parameters in diabetes and or hypercholesterolemia but were still above the normal values.
Scanning electron microscopic observation of lens
Control or fish oil-supplementation possessed regularly oriented lens fibers with intact fixed ball and socket ( Figures 1A and 1B). Diabetes and or hypercholesterolemia exhibited disorganized and loosely attached lens fibers with increased deformation of ball and socket (Figures 1C, 1E and 1G). Fish oil-supplementation to diabetes and or hypercholesterolemia improved the induced lens lesions and resorted the structural integrity of lens fibers ( Figures 1D, 1F and 1H).
Figure 1: Scanning electron microscopy of lens. (A&B) Control and fish-oil supplementation showing normal integrity of lens fibers and ball and sockets. (C) Diabetes showing disorganized lens fibers and deformed ball and sockets. (D) Diabetes and fish oil supplementation showing improvements. (E) Hypercholesterolemia showing widened lens fibers and deformed ball and sockets. (G) Diabetes and hypercholesterolemia showing deformation of ball and sockets and degenerated lens fibers. (H) Diabetes and hypercholesterolemia and fish oil showing improvements. Abbreviations: DBS: deformed ball and sockets; NBS: Normal ball and sockets
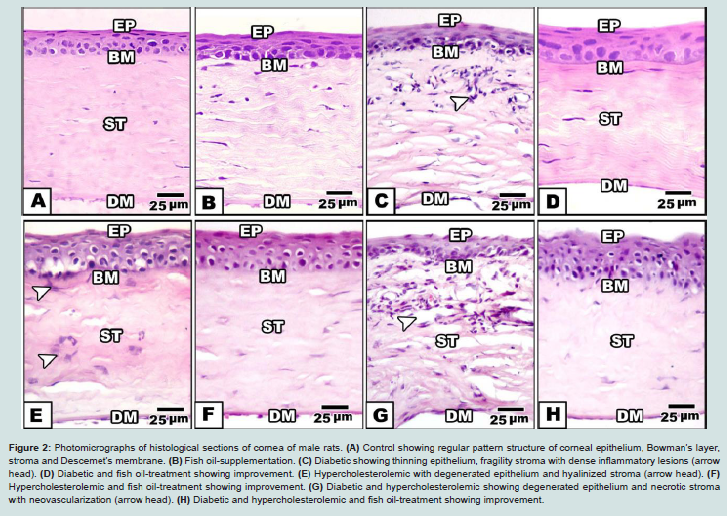
Control and fish-oil supplemented male rats showed normal pattern structure of cornea. Externally, it is formed of normally oriented non-keratinized peripheral stratified epithelium. Its basal columnar or cubical cells are resting on basement membrane. Thin sheath of Bowman’s layer is detected underneath the epithelium. The corneal stroma occupied a large space and composed of regular arrangement of collagen fibrils fenestrated by keratocytes. Transparency of cornea is related to its organized pattern arrangement. At the end of stroma, Descemet’s membrane with underlying endothelium is remarked ( Figures 2A and 2B).
Figure 2: Photomicrographs of histological sections of cornea of male rats. (A) Control showing regular pattern structure of corneal epithelium, Bowman’s layer, stroma and Descemet’s membrane. (B) Fish oil-supplementation. (C) Diabetic showing thinning epithelium, fragility stroma with dense inflammatory lesions (arrow head). (D) Diabetic and fish oil-treatment showing improvement. (E) Hypercholesterolemic with degenerated epithelium and hyalinized stroma (arrow head). (F) Hypercholesterolemic and fish oil-treatment showing improvement. (G) Diabetic and hypercholesterolemic showing degenerated epithelium and necrotic stroma with neovascularization (arrow head). (H) Diabetic and hypercholesterolemic and fish oil-treatment showing improvement.Abbreviations: Ep: Epithelium; BM: Basement membrane; St: Stroma; DM: Descemet’s membrane. H&E
In hypercholesterolemic group, there was a detected vacuolar degeneration of epithelial lining cells, hyalinization of collagenous stroma and Bowman’s layer. Necrotic foci were detected with apparent degeneration of keratocytes (Figure 1E ).
In experimental diabetic and / or hypercholesterolemic group supplemented fish oil, there was apparent amelioration especially of the peculiar structure of corneal epithelium. The stroma restored almost the keratocyte arrangement (Figures 1D, 1F and 1H).
At ultrastructural level, the control possessed varying epithelialstructures being flattened in the superficial layers with characteristic micrvilli in their peripheral marginal cells. The deep corneal epithelium forms the so-called wing cells, having polygonal shape with ovoid indented nucleus. Their basal lamina appeared folded. Underneath the corneal epithelium, the Bowman’s layer is formed of collagenous fibrils. The stroma possessed regularly oriented parallel fibrils. Keratocytes are dispersed in between the stroma and communicated with each other by their branching processes. Descemet’s membrane is composed of varying degree of collagenous densities and enclosed internally by the endothelial lining layer (Figures 2A and 2C).
In experimentally diabetic group, there was a detected damaged epithelial cell, abnormal stroma with pyknotic keratocytes and massive degeneration of deep stromal layer and deteriorated Descemet’s membrane and endothelial lining layer (Figures 2A1 and 2C1). Fish oil supplementation to diabetic group, restored normal pattern structure of epithelium, stroma and Descemet’s membrane (Figures 2A2 and 2C2).
Experimental hypercholesterolemic group revealed massive necrosis of epithelial lining cells, disorganized stroma with apparent necrosis of deep layer and degeneration of endothelial layer (Figures 3A-3C ). Fish oil-supplementation improved the elementary structure of corneal layers (Figures 3A1-3C1).
Figure 3: Transmission electron micrograph of cornea of male rats. A-C. Control showing normal epithelium (A) regular stroma with keratocytes (B) and Descemet’s membrane (C) A1-C1. Diabetes showing damaged epithelium with distorted basal lamina (A1), damaged keratocytes within stroma (B1) and degenerated of both stroma and Descemet’s membrane (C1) Arrow head showed folded basal lamina. A2-C2. Diabetic and fish oil-treatment showing partial improvement. Abbreviation: BM: Basement membrane; BL: Basal lamina; DM: Descemet’s membrane; DDM: degenerated Descemet’s membrane; DK: Degenerated keratocytes; Ep: Epithelium; K: keratocytes; St: Stroma; Ma: Macrophage.
Figure 4: Transmission electron micrographs of cornea of male rats. A-C. Hypercholesterolemic showing vacuolar degenerated and pyknotic epithelium (A & Aa), distorted stroma with damaged keratocytes (B) and degenerated Descemet’s membrane (C) Arrow showing degenerated epithelium. A1-C1. Hypercholesterolemia and fish oil-treatment showing partial improvement. Abbreviation; BM: Basement Membrane; DM: Descemet’s Membrane; DDM: Degenerated Descemet’s Membrane; DK: Degenerated Keratocytes; DEP: Degenerated Epithelium; DST: Degenerated Stroma; EP: Epithelium; K: Keratocytes; ST: Stroma; Ma: Macrophage.
Discussion
The observed findings revealed apparent increase of lenticular contents of protein carbonylation and glycation end products. Similar findings were reported by Zarina et al. [33]. Diabetic related cataractous lenses were found to possess apparent increase of carbonylated lens proteins [34]. Increased glycosylation of lens epithelial basement membranes was reported in diabetic patients and adult diabetic rats neonatal streptozotocin induced rat model [35-39] The elevated levels of AGEs in lens of diabetic rat were markedly correlated with apoptosis of lens epithelium and apparent depletion of epithelial cells within affected lenses [40,41]. Øsnes-Ringen et al. observed DNA strand breaks and increased level of oxidized purines in cataractous lenses [42].The observed findings detected marked increase of endothelin-1 and adhesion molecules (ICAM-1 & VCAM-1) in diabetic and/ or hypercholesterolemic lenses. Endothelin-1 (ET-1) was found to cause inhibition of lens active Na-K transport, keeping water balance for promoting lens function. Activation of ET receptors led to marked increase of cytoplasmic calcium concentration in cultured lens epithelial cells, the markers of cataracts [43]. Increased lens calcium content was detected in aged related rat cataract formation [44]. It was found that increase lenticular calcium content led to altered protein configuration through induction of disulfide and dityrosine covalent cross-linking and the formation of peroxynitrite, the markers of cataract [45].
Adhesion molecules are a cell surface glycoprotein, overexpressed in lens epithelium by increased fructose level especially, ICAM-1 [46]. Increased expression of ICAM-1, led to a decrease in lens epithelial proliferation and consequently plays a great role in progression of diabetic cataract [47]. Klein et al. mentioned that ICAM-1 had a great role in lenticular inflammation and developing of aging-related nuclear cataract [48].
The observed findings revealed apparent increase of zinc and iron content in diabetic and /or hypercholesterolemic lens. Similar findings of increased lens zinc and iron content were reported by Dawczynski et al. in human cataractous lenses. Zinc was found to be increased in diabetic and cataractous lenses [49-51]. As we know that the superoxide dismutase (SOD) is dependent upon zinc and copper ions for promoting its activity and scavenging superoxide anion which is important part in oxidative stress. Increased zinc content seemed to be attributed to disrupted lens metabolism. Iron was found to be markedly increased in diabetic lens [52]. Concerning iron, Transferrin and Fe concentrations was markedly at higher level in the intraocular fluids in diseased conditions and tend to be accumulated in lens during ocular inflammation. There are two ways of picking iron by tissues, receptor-medicated endocytosis of diferric transferrin and cell membrane mediated by an oxido-reductase [53].
The observed increase of biochemical markers were confirmed by the degeneration , disrupted orientation of lens fibers and deformation of ball and socket in diabetic or hypercholesterolemic rats. Fish oil-supplementation improved the marked increase of carbonylated and glycated lens proteins, adhesion molecules and zinc and iron contents.
Weikel et al. reported that cataractous lenses can be reduced by feeding on diets rich in omega-3 fatty acids in diabetic mice [7]. Also, dietary intakes of omega-3 polyunsaturated fatty acids may resolute the incidence of nuclear cataract in patients [54]. The ameliorating role of fish oil may be attributed to its antioxidant property and presence of fat-soluble vitamins (A, D and E), polyunsaturated fatty acids, sterols and mild amounts of beta carotene as mentioned by Luterotti et al. in cod liver oil [55].
Concerning cornea, which is present in front of lens and have important role in protection of lens and preserving visual activity, Histo-cytological observations of diabetes and or hypercholesterolemia group revealed apparent damage of corneal epithelium with either vacuolated cytoplasm or pyknotic nuclei. There was a detected hyalinization and degeneration of both Bowman’s and stromal collagenous layers as well as of stromal keratocytes. Multifocal or diffuse leukocytic inflammatory cells aggregation was detected in the superficial collagenous stromal layers. In case of diabetic and hypercholesterolemic group, there was a detected increase of neovascularization explained by numerous spaced regions outlined internally by flattened cells suspected endothelial cells as well as degeneration of the endothelium lining the Descemet’s membrane (DM). Hypercholesterolemic showed the least damage comparing with diabetes and or hypercholesterolemia (Figures 2-5).
Figure 5: Transmission electron micrographs of cornea of male rats. A-C. Diabetes and hypercholesterolemia showing degenerated and pyknotic epithelium (A) degenerated collagenous stroma and keratocytes (B) and damaged Descemet’s membrane and ruptured endothelium (C) A1-C1. Diabetes and hypercholesterolemia and fish oil-treatment showing partial improvement. Arrow head indicated folded basal lamina of epithelial layer and slight vacuolation of collagenous stroma.Abbreviations: BM: Basement Membrane; DM: Descemet’s Membrane; DDM: Degenerated Descemet’s Membrane; DK: Degenerated Keratocytes; DEP: Degenerated Epithelium; DST: Degenerated Stroma; EP: Epithelium; HD: Hypercholesterolemia and Diabetes; HDF: Hypercholesterolemia and Diabetes and Fish oil; K: Keratocytes; PN: Pyknotic Nuclei; ST: Stroma.
Diabetic patients, KKAy mice, rat and monkey exhibited pronounced alterations in corneal epithelium, fragility of collagenous stroma and degeneration of keratocytes [57-60]. Friend et al. mentioned that diabetic rats possessed apparent degeneration of basal epithelial cells associated with thickening and folding of their basement membrane leading to increase sorbitol pathway products. Altered endothelium may interfere with the maintaining transparency of stroma as a result of increased edematous lesions as detected by hyalinization and disrupted vision [61-63].
In experimental diabetic and or hypercholesterolemic group supplemented fish oil, there was apparent amelioration especially of the peculiar structure of corneal epithelium. The stroma restored almost the keratocyte arrangement. In vitro cultures of both cornea endothelial and lens epithelial cells with 10 mg/ml cholestarol revealed apparent increase of apoptosis and DNA fragmentation associated with over expression of interleukin-1b-converting enzyme and CPP32 proteases [64]. Mitochondria represent the main integral part of corneal lens epithelium. Damage of lens epithelium in contributed to the mitochondrial damage and consequently increased the liberation of free radicals and intern affected the corneal region [65].
Fish oil supplementation was found to enhanced healing of corneal damage induced by scopolamine as well as of photoreactive keratectomy and improve corneal innervation in streptozotocin treated mice [66-68].
The authors finally concluded that diabetes and or hypercholesterolemia interfered with degeneration and disruption of lens fibers assessed by increase lenticular biomarkers and associated with corneal damage. Fish oil supplementations scavenge free radicals and ameliorated both lens and cornea.
References
- Miranda M, Araiz J, Romero FJ (2013) Lutein and diabetic cataracts. In: Watson RR and Preedy VR (Eds). Bioactive food as dietary interventions for diabetes. San Diego Academic Press, USA, pp. 275-289.
- Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 5: 150-159.
- El-Sayyad HI, El-Sherbiny MA, Sobh MA, Abou-El-Naga AM, Ibrahim MA, et al. (2011) Protective effects of Morus alba leaves extract on ocular functions of pups from diabetic and hypercholesterolemic mother rats. Int J Biol Sci 7: 715-728.
- Gerencser AA (2015) Bioenergetic analysis of single pancreatic betacells indicates an impaired metabolic signature in type 2 diabetic subjects. Endocrinology 156: 3496-3503.
- El-Sayyad HI, El-Mansi AA, Bakr IH (2015) Hypercholesterolemia induced ocular disorder: Ameliorating role of phytotherapy. Nutrition 31: 1307-1316.
- Weikel KA, Garber C, Baburins A, Taylor A (2014) Nutritional modulation of cataract. Nutr Rev 72: 30-47.
- Hegde KR, Varma SD (2005) Prevention of cataract by pyruvate in experimentally diabetic mice. Mol Cell Biochem 269: 115-120.
- Hashim Z, Zarina S (2012) Osmotic stress induced oxidative damage: possible mechanism of cataract formation in diabetes. J Diabetes Complications 26: 275-279.
- Leino M, Pyörälä K, Lehto S, Rantala A (1992) Lens opacity in patients with hypercholesterolemia and ischemic heart disease: Electronic lens opacity measurements. Doc Ophthalmol 80: 309-315.
- Pokupec R, Kalauz M, Turk N, Turk Z (2003) Advanced glycation end products in human diabetic and non-diabetic cataractous lenses. Graefes Arch Clin Exp Ophthalmol 241: 378-384.
- Nishimoto JH, Townsend JC, Selvin GJ, De Land PN (1990) Corneal arcus as an indicator of hypercholesterolemia. J Am Optom Assoc 61: 44-49.
- Zech LA Jr, Hoeg JM (2008) Correlating corneal arcus with atherosclerosis in familial hypercholesterolemia. Lipids Health Dis 7: 7.
- Kruth HS (1987) Accumulation of unesterified cholesterol in limbal cornea and conjunctiva of rabbits fed a high-cholesterol diet. Detection with filipin. Atherosclerosis 63: 1-6.
- Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, et al. (2014) Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci 55: 3603-4615.
- Yorek MS, Obrosov A, Shevalye H, Holmes A, Harper MM, et al. (2015) Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J Peripher Nerv Syst 20: 24-31.
- Tiutiuca C (2013) Assessment of central corneal thickness in children with diabetus mellitus type I. Oftalmologia 57: 26-32.
- Priyadarsini S, Sarker-Nag A, Allegood J, Chalfant C, Karamichos D (2014) Description of the sphingolipid content and subspecies in the diabetic cornea. Curr Eye Res 40: 1204-1210.
- Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H (2014) Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med 31: 431-438.
- Pollreisz A, Schmidt-Erfurth U (2010) Diabetic cataract-pathogenesis, epidemiology and treatment. J Ophthalmol 2010: 608751.
- Adnan X, Suheimat M, Efron N, Edwards K, Pritchard N, et al. (2015) Biometry of eyes in type 1 diabetes. Biomed Opt Express 6: 702-715.
- Reddy VS, Reddy GB (2015) Role of crystallins in diabetic complications. Biochim Biophys Acta 269-277.
- Reddy VS, Kumar CU, Reddy GB (2014) Effect of chronic hyperglycemia on crystallin levels in rat lens. Biochem Biophys Res Commun 446: 602-607.
- Su S, Leng F, Guan L, Zhang L, Ge J, et al. (2014) Differential proteomic analyses of cataracts from rat models of type 1 and 2 diabetes. Invest Ophthalmol Vis Sci 55: 7848-7861.
- Reddy PY, Giridharan NV, Reddy GB (2012) Activation of sorbitol pathway in metabolic syndrome and increased susceptibility to cataract in Wistar-Obese rats. Mol Vis 18: 495-503.
- Girão H, Mota MC, Ramalho J, Pereira P (1998) Cholesterol oxides accumulate in human cataracts. Exp Eye Res 66: 645-652.
- Jacob RF, Cenedella RJ, Mason RP (2001) Evidence for distinct cholesterol domains in fiber cell membranes from cataractous human lenses. J Biol Chem 276: 13573-13578.
- Perales S, Alejandre MJ, Palomino-Morales R, Torres C, Iglesias J, et al. (2009) Effect of oxysterol-induced apoptosis of vascular smooth muscle cells on experimental hypercholesterolemia. J Biomed Biotechnol 2009: 456208.
- Sengupta A, Ghosh M (2013) Protective effect of eicosapentaenoic aciddocosahexaenoic acid and alpha-linolenic acid rich phytosterol ester on brain antioxidant status and brain lipid composition in hypercholesterolemic rats. Indian J Exp Biol 51: 241-248.
- Sekhon-Loodu S, Catalli A, Kulka M, Wang Y, Shahidi F, et al. (2014) Apple flavonols and n-3 polyunsaturated fatty acid-rich fish oil lowers blood C-reactive protein in rats with hypercholesterolemia and acute inflammation. Nutr Res 34: 535-543.
- Povoski SP, McCullough PJ, Zhou W, Bell RH Jr (1993) Induction of diabetes mellitus in Syrian golden hamsters using stored equilibrium solutions of streptozotocin. Lab Anim Sci 43: 310-314.
- Enkhmaa B, Shiwaku K, Anuurad E, Nogi A, Kitajima K, et al. (2005) Prevalence of the metabolic syndrome using the Third Report of the National Cholesterol Educational Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) and the modified ATP III definitions for Japanese and Mongolians. Clin Chim Acta 352: 105-113.
- Scancar J, Milacic R, Benedik M, Bukovec P (2000) Determination of trace elements and calcium in bone of the human iliac crest by atomic absorption spectrometry. Clin Chim Acta 293: 187-192.
- Zarina S, Zhao HR, Abraham EC (2000) Advanced glycation end products in human senile and diabetic cataractous lenses. Mol Cell Biochem 210: 29-34.
- Olofsson EM, Marklund SL, Behndig A (2009) Enhanced diabetes-induced cataract in copper-zinc superoxide dismutase-null mice. Invest Ophthalmol Vis Sci 50: 2913-2918.
- Shin DH, Mandel SS, Lee JH, Ernst B, Newman BL, et al. (1994) Increased glycosylation of human lens epithelial basement membrane in diabetes mellitus. Korean J Ophthalmol 8: 26-31.
- Balog Z, Klepac R, Sikic J, Jukic-Lesina T (2001) Protein carbonylation and glycation in human lenses. Coll Antropol 25: 145-148.
- Hashim Z, Zarina S (2011) Advanced glycation end products in diabetic and non-diabetic human subjects suffering from cataract. Age (Dordr) 33: 377- 384.
- Muthenna P, Akileshwari C, Saraswat M, Bhanuprakash Reddy G (2012) Inhibition of advanced glycation end-product formation on eye lens protein by rutin. Br J Nutr 107: 941-949.
- Patil MA, Suryanarayana P, Putcha UK, Srinivas M, Reddy GB (2014) Evaluation of neonatal streptozotocin induced diabetic rat model for the development of cataract. Oxid Med Cell Longev 2014: 463264.
- Kim J, Kim CS, Sohn E, Kim H, Jeong IH, et al. (2010) Lens epithelial cell apoptosis initiates diabetic cataractogenesis in the Zucker diabetic fatty rat. Graefes Arch Clin Exp Ophthalmol 248: 811-818.
- Tseng SH, Yen JS, Chien HL (1994) Lens epithelium in senile cataract. J Formos Med Assoc 93: 93-98.
- Øsnes-Ringen O, Azqueta AO, Moe MC, Zetterström C, Røger M, et al. (2013) DNA damage in lens epithelium of cataract patients in vivo and ex vivo. Acta Ophthalmol 91: 652-656.
- Okafor MC, Delamere NA (2001) The inhibitory influence of endothelin on active sodium-potassium transport in porcine lens. Invest Ophthalmol Vis Sci 42: 1018-1023.
- El-Sayyad HI, Khalifa SA, El-Sayyad FI, Maylod EE (2012) Biochemical and ultra structural changes in lens during aging albino rats. Br J Med Med Res 2: 105-121.
- Ghahramani M, Yousefi R, Khoshaman K, Alavianmehr MM (2015) The impact of calcium ion on structure and aggregation propensity of peroxynitritemodified lens crystallins: new insights into the pathogenesis of cataract disorders. Colloids Surf B Biointerfaces 125: 170-180.
- Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, et al. (2008) Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol 19: 1712-1720.
- Fan H, Suzuki T, Ogata M, Nakagawa Y, Onouchi H, et al. (2012) Expression of PCNA, ICAM-1, and vimentin in lens epithelial cells of cataract patients with and without Type 2 Diabetes. Tokai J Exp Clin Med 37: 51-56.
- Klein BE, Klein R, Lee KE, Knudtson MD, Tsai MY (2006) Markers of inflammation, vascular endothelial dysfunction, and age-related cataract. Am J Ophthalmol 141: 116-122.
- Dawczynski J, Blum M, Winnefeld K, Strobel J (2002) Increased content of zinc and iron in human cataractous lenses. Biol Trace Elem Res 90: 15-23.
- Gündüz G, Gündüz F, Yucel i, Sentürk UK (2003) Levels of zinc and magnesium in senile and diabetic senile cataractous lenses. Biol Trace Elem Res 95: 107-112.
- Srivastava VK, Varshney N, Pandey DC (1992) Role of trace elements in senile cataract. Acta Ophthalmol (Copenh) 70: 839-841.
- Cekic O, Bardak Y (1998) Lenticular calcium, magnesium, and iron levels in diabetic rats and verapamil effect. Ophthalmic Res 30: 107-112.
- McGahan MC, Grimes AM, Nasisse MP, Fleisher LN (1995) Iron uptake by cultured lens epithelial cells. Graefes Arch Clin Exp Ophthalmol. 233: 354-359.
- Townend BS, Townend ME, Flood V, Burlutsky G, Rochtchina E, et al. (2007) Dietary macronutrient intake and five-year incident cataract: the blue mountains eye study. Am J Ophthalmol 143: 932-939.
- Luterotti S, Franko M, Bicanic D (1999) Ultrasensitive determination of betacarotene in fish oil-based supplementary drugs by HPLC-TLS. J Pharm Biomed Anal 21: 901-909.
- El-Sayyad HI, Elmansi A, Guida MS, Mohammed EA (2015) Markers characterizing corneal damage during aging of rat. J Adv Chem 11: 3532-3539.
- Bikbova G, Oshitari T, Tawada A, Yamamoto S (2012) Corneal changes in diabetes mellitus. Curr Diabetes Rev 8: 294-302.
- Watanabe H, Katakami C, Miyata S, Negi A (2002) Corneal disorders in KKAy mouse: a type 2 diabetes model. Jpn J Ophthalmol 46: 130-139.
- Take G, Karabay G, Erdogan D, Duyar I (2006) The ultrastructural alterations in rat corneas with experimentally-induced diabetes mellitus. Saudi Med J 27: 1650-1655.
- Zou C, Wang S, Huang F, Zhang YA (2012) Advanced glycation end products and ultrastructural changes in corneas of long-term streptozotocin-induced diabetic monkeys. Cornea 31: 1455-1459.
- Friend J, Ishii Y, Thoft RA (1982) Corneal epithelial changes in diabetic rats. Ophthalmic Res 14: 269-278.
- Waring GO 3rd, Bourne WM, Edelhauser HF, Kenyon KR (1982) The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 89: 531-590.
- Hillenar I, van Cleynenbreugel H, Remeijer L (2012) How normal is the transparent cornea? Effects of aging on corneal morphology. Ophthalmology 119: 241-248.
- Inoue K, Kubota S, Tsuru T, Araie M, Seyama Y (2000) Cholestanol induces apoptosis of corneal endothelial and lens epithelial cells. Invest Ophthalmol Vis Sci 41: 991-997.
- Babizhayev MA, Yegorov YE (2010) Reactive oxygen species and the aging eye: Specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract-a novel platform of mitochondria-targeted antioxidants with broad therapeutic potential for Redox regulation and detoxification of oxidants in eye diseases. Am J Ther 23: e98-e117.
- de Paiva CS, Schwartz CE, Gjörstrup P, Pflugfelder SC (2012) Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea 31: 1299-1303.
- Ong NH, Purcell TL, Roch-Levecq AC, Wang D, Isidro MA, et al. (2013) Epithelial healing and visual outcomes of patients using omega-3 oral nutritional supplements before and after photorefractive keratectomy: a pilot study. Cornea 32: 761-715.
- Shevalye H, Yorek MS, Coppey LJ, Holmes A, Harper MM, et al. (2015) Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J Neurophysiol 114: 199-208.