Journal of Hematology & Thrombosis
Download PDF
Research Article
Assessment of GeneticsMutations in Genes CDAN1, SEC23B and Dell-15q22 in Inducate Congenital Dyserythropoietic Anemia Syndrome
Shahin Asadi*
- Department of Medical Genetics, Harvard University of Medical Sciences, USA
*Address for Correspondence: Shahin Asadi, Department of Medical Genetics, Director of the Division of Medical Genetics and Molecular Pathology Research, Harvard University of Medical Sciences, Massachusetts, USA. E-mail: shahin.asadi1985@gmail.com
Citation: Asadi S. Assessment of Genetics Mutations in Genes CDAN1, SEC23B and Dell-15q22 in Inducate Congenital Dyserythropoietic Anemia Syndrome. J Hematol Thromb 2019;4(1): 3.
Copyright © 2019 Asadi S, et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis| ISSN: 2380-6842 | Volume: 4, Issue: 1
Submission: 02 March, 2019, 2018 | Accepted: 12 April, 2019 | Published: 16 April, 2019.
Abstract
CDA syndrome is a genetic disorder that affects the growth of red blood cells. This syndrome is one of the anemia disorders characterized by a lack of red blood cells. Researchers have identified three types of CDA syndromes, each of which has different genetic causes and patterns of signs and symptoms. Type 1 CDA syndrome is characterized by moderate to severe anemia. CDA1 syndrome is caused by the mutation of the CDAN1 gene, which is based on the long arm of chromosome 15 as 15q15.2. The CDA2 syndrome is caused by the mutation of the SEC23B gene, which is based on the short arm of chromosome number 20, 20p11.23.
Keywords
Generalized congenital dysrhythmia anemia syndrome; CDAN1; SEC23B genes; Blood disorders
Generalized Congenital Dysrhythmia Anemia Syndrome (CDA)
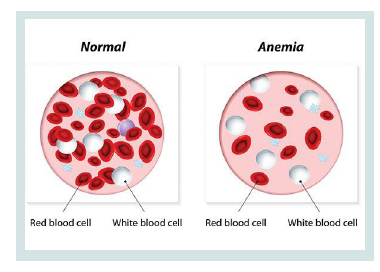
CDA syndrome is a genetic disorder that affects the growth of red blood cells. This syndrome is one of the anemia disorders characterized by a lack of red blood cells. This deficiency prevents the transfer of oxygen from the blood to the tissues of the body. Symptoms include fatigue, weakness, dullness, and other complications [1] (Figure 1).
Clinical Signs of Congenital Dysrhythmia Anemia Syndrome (CDA)
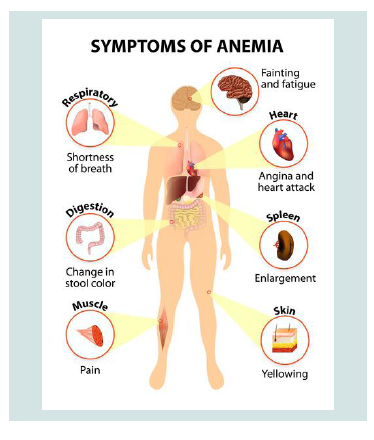
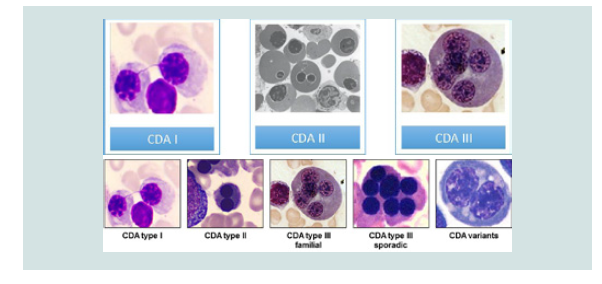
Researchers have identified three types of CDA syndromes, each of which has different genetic causes and patterns of signs and symptoms. Type 1 CDA syndrome is characterized by moderate to severe anemia1. This disorder is usually diagnosed in childhood or adolescence, although in some cases the syndrome may also be diagnosed before birth. Many people with CDA1 syndrome experience jaundice in the skin and the white bowel of the eye, and the size of the liver and spleen (hepatosplenomegaly). This disorder also causes excessive iron intake to the body, causing damage to the tissues and organs. In particular, excess iron can lead to abnormal heart rhythm (arrhythmia), congestive heart failure, diabetes, and chronic liver disease (cirrhosis). Rarely, people with CDA1 syndrome are born with skeletal disorders, which often include fingers or toes [2] (Figure 2).
Anemia associated with CDA2 syndrome varies from mild to severe, and most people with this disorder, jaundice, enlarged liver and spleen (hepatocellular membranes) and gallstones. CDA2 syndrome is usually diagnosed in adolescence or childhood. Abnormal iron deficiency usually occurs after the age of 20, leading to complications such as heart disease, diabetes and cirrhosis of the liver [3].
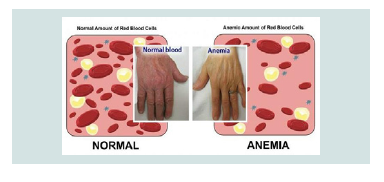
Symptoms and Symptoms of CDA3 Syndrome are different from the two previous ones. Adults with CDA3 syndrome have retinal disturbances that can cause visual impairment. Some people with CDA3 syndrome have a blood disorder called monoclonal gomopathy that can lead to white blood cell (multiple myeloma) cancer [4] (Figure 3).
Etiometry of Congenital Dysrhythmia Anemia syndrome (CDA)
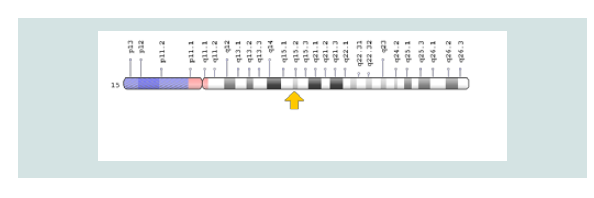
CDA1 syndrome is caused by the mutation of the CDAN1 gene, which is based on the long arm of chromosome 15 as 15q15.2. The function of this gene is still not well understood, and it is unclear how the mutation in this gene leads to the signs and symptoms of CDA1 syndrome.
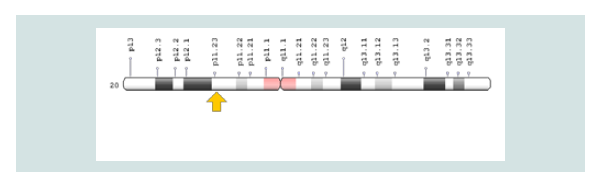
The CDA2 syndrome is caused by the mutation of the SEC23B gene, which is based on the short arm of chromosome number 20, 20p11.23. The gene provides instructions for protein synthesis that interfere with the transport of other proteins within the cells. During the growth of red blood cells, the protein produced from the SEC23B gene may help to ensure that proteins are transferred to the required sites. Researchers are trying to determine which mutations in the SEC23B gene lead to symptoms and symptoms of CDA2 syndrome [5] (Figure 4 and 5).
Figure 4: Schematic view of Chromosome No. 15 in which the CDAN1 gene is located in the long arm of this chromosome 15q15.2.
Figure 5: Schematic view of chromosome number 20 where SEC23B gene is based on the short arm of this chromosome as 20p11.23.
The genetic cause of CDA3 syndrome has not yet been determined. This syndrome is probably caused by a mutation in a gene in the long arm of chromosome 15 in the 15q22 region. The genetic changes responsible for the development of CDA syndrome cause disturbances in the normal growth of red blood cells, known as the erythropoiesis process. The term disperitopoietic is a condition in which red blood cells form abnormally. In people with CDA, abnormal red blood cells are called erythroblasts in an unusual form, and other disorders are like additional nuclei. These abnormal erythroblasts cannot be converted to adult red blood cells. Therefore, deficiency of healthy red blood cells leads to signs and symptoms of anemia, as well as histoplasmosis and abnormal iron elevation [6] (Figure 6).
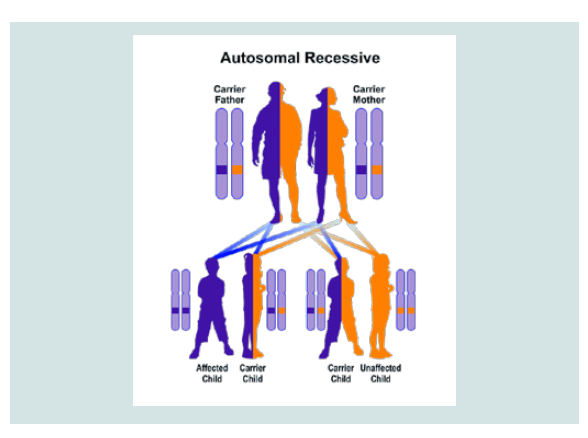
CDA1 and CDA2 syndrome follow an autosomal recessive hereditary pattern. Therefore, in order to produce this syndrome, two copies of the mutant gene CDAN1 and SEC23B (one of the fathers and the other of the mother) are needed and the chance of having a child with autosomal recessive syndrome is 25% for each pregnancy [7].
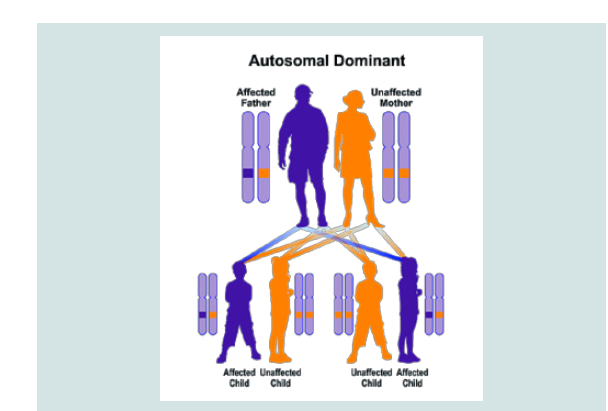
CDA3 syndrome follows the dominant autosomal inheritance pattern. Therefore, in order to create this syndrome, a copy of the unrelated mutation gene (parent or parent) is required and the chance of having a child with this syndrome in the dominant autosomal state is 50% for each possible pregnancy [8].
Frequency of congenital diarticular anemia syndrome (CDA)
CDA2 syndrome is the most common form of this disorder, and so far, more than 300 cases of CDA2 syndrome have been reported from medical literature throughout the world. A rare CDA3 syndrome is reported in only a few families in Sweden, Argentina and the United States. Frequency of CDA1 syndrome is unknown. Because CDA syndrome is very rare and its signs and symptoms indicate overlap with other disorders, so many cases are unlikely to be diagnosed or indirectly diagnosed as other disorders [9] (Figure 7).
Figure 7: Schematic view of Autosomal recessive hereditary pattern followed by CDA1 and CDA2 syndromes.
Congenital Dyserythropoietic Anemia Syndrome (CDA)
CDA syndrome is diagnosed based on the clinical, clinical and physical findings of the patients and some pathological tests. The most accurate method for detecting this syndrome is the molecular genetic testing of the CDAN1 and SEC23B genes to investigate the presence of possible mutations [10] (Figure 8).
Congenital Dyserythropoietic Anemia Syndrome (CDA)
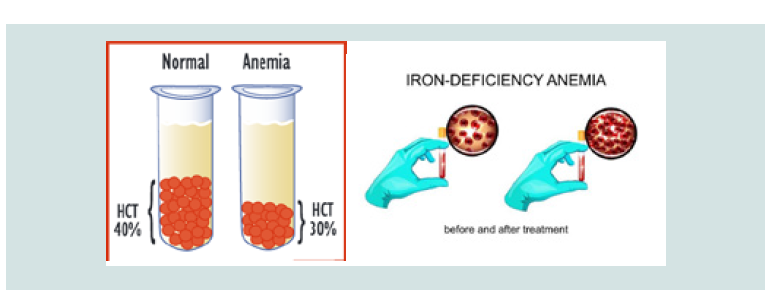
The CDA syndrome treatment and management strategy is symptomatic and supportive. Treatment may be done by a team of experts, including pediatricians, eye specialists, cardiologists, liver specialists, hematologists, dietitians and other healthcare professionals. There is no valid treatment for this syndrome, and all clinical measures are needed to reduce the suffering of the sufferers. Genetic counselling is also a special place for all parents who want a healthy baby [11] (Figure 9).
Figure 9: Schematic of Hematocrit volume (HCT) in a healthy person and an individual with anemia before and after treatment.
Discussion and Conclusion
(CDA syndrome is a genetic disorder that affects the growth of red blood cells. This syndrome is one of the anemia disorders characterized by a lack of red blood cells. Researchers have identified three types of CDA syndromes, each of which has different genetic causes and patterns of signs and symptoms. Type 1 CDA syndrome is characterized by moderate to severe anemia. CDA1 syndrome is caused by the mutation of the CDAN1 gene, which is based on the long arm of chromosome 15 as 15q15.2. The CDA2 syndrome is caused by the mutation of the SEC23B gene, which is based on the short arm of chromosome number 20, 20p11.23. There is no valid treatment for this syndrome, and all clinical measures are needed to reduce the suffering of the sufferers.
References
- Denecke J, Marquardt T (2009) Congenital dyserythropoietic anemia type II (CDAII/HEMPAS): where are we now? Biochim Biophys Acta 1792: 915-20.
- Dgany O, Avidan N, Delaunay J, Krasnov T, Shalmon L, et al. (2002) Congenital dyserythropoietic anemia type I is caused by mutations in codanin-1. Am J Hum Genet 71: 1467-1474
- Heimpel H, Anselstetter V, Chrobak L, Denecke J, Einsiedler B, et al. (2003) Congenital dyserythropoietic anemia type II: epidemiology, clinical appearance, and prognosis based on long-term observation. Blood 102: 4576-4581.
- Heimpel H, Schwarz K, Ebnother M, Goede JS, Heydrich D, et al. (2006) Congenital dyserythropoietic anemia type I (CDA I): molecular genetics, clinical appearance, and prognosis based on long-term observation. Blood 107: 334-340.
- Heimpel H (2004) Congenital dyserythropoietic anemias: epidemiology, clinical significance, and progress in understanding their pathogenesis. Ann Hematol 83: 613-621
- Renella R, Wood WG (2009) The congenital dyserythropoietic anemias. Hematol Oncol Clin North Am 23: 283-306.
- Sandstrom H, Wahlin A (2000) Congenital dyserythropoietic anemia type III. Haematologica 85: 753-757.
- Schwarz K, Iolascon A, Verissimo F, Trede NS, Horsley W, et al. (2009) Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet 41: 936-940.
- Tamary H, Dgany O, Proust A, Krasnov T, Avidan N, et al. (2005) Clinical and molecular variability in congenital dyserythropoietic anaemia type I. Br J Haematol 130: 628-634.
- Tamary H, Dgany O (2016) Congenital Dyserythropoietic Anemia Type I. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, et al. (Eds). GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017.
- Wickramasinghe SN, Wood WG (2005) Advances in the understanding of the congenital dyserythropoietic anaemias. Br J Haematol 131: 431-446.