Journal of Hematology & Thrombosis
Download PDF
Research Article
*Address for Correspondence: Roy E. Smith, Division of Hematology/Oncology, University of Pittsburgh Medical Center, 5150 Centre Avenue, Pittsburgh, PA 15232, USA, Tel: 412-648-6466; Fax: 412-648-6579; E-mail: smithre@upmc.edu
Citation: Tuchayi AM, Sheth S, Smith RE. The Evaluation of Cardiac Disorders and Systemic Hypertension in JAK2V617F Positive Patients at a Multicenter Institution. J Hematol Thromb 2016;2(1): 4.
Copyright © 2016 Smith, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 2, Issue: 1Submission: 11 May, 2016 | Accepted: 23 June, 2016 | Published: 29 June, 2016
Reviewed & Approved by: Dr. Raul H. Morales-Borges, American Red Cross in San Juan, Practices, Ashford Institute of Hematology & Oncology, USA
Sixteen patients had either embolic or ischemic stroke. No patients had hemorrhagic stroke. Among these patients 11 patients were JAK2 V617F positive while 5 patients were JAK2 V617F negative. Eleven Patients had MI. Eight patients were JAK2 V617F positive while 3 patients were JAK2 V617F negative. Fifteen patients had PAD. Among these patients 12 patients were JAK2 V617F positive while 3 patients were JAK2 V617F negative. Fifteen patients had HF. Among these patients 12 patients were JAK2 V617F positive, and 3 patients were JAK2 V617F negative.
The Evaluation of Cardiac Disorders and Systemic Hypertension in JAK2V617F Positive Patients at a Multicenter Institution
Abuzar Moradi Tuchayi1, Siddharth Sheth2 and Roy E. Smith1*
- 1Division of Hematology/Oncology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
- 2Division of General Internal Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
*Address for Correspondence: Roy E. Smith, Division of Hematology/Oncology, University of Pittsburgh Medical Center, 5150 Centre Avenue, Pittsburgh, PA 15232, USA, Tel: 412-648-6466; Fax: 412-648-6579; E-mail: smithre@upmc.edu
Citation: Tuchayi AM, Sheth S, Smith RE. The Evaluation of Cardiac Disorders and Systemic Hypertension in JAK2V617F Positive Patients at a Multicenter Institution. J Hematol Thromb 2016;2(1): 4.
Copyright © 2016 Smith, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology & Thrombosis | ISSN: 2380-6842 | Volume: 2, Issue: 1Submission: 11 May, 2016 | Accepted: 23 June, 2016 | Published: 29 June, 2016
Reviewed & Approved by: Dr. Raul H. Morales-Borges, American Red Cross in San Juan, Practices, Ashford Institute of Hematology & Oncology, USA
Abstract
In this retrospective study; we evaluate cardiac disorders and systemic hypertension in JAK2 V617F positive patients. We identified 1000 patients in our hospital system that had been evaluated for JAK2 V617F mutation. Among these patients we found 100 JAK2 V617F positive patients and 100 JAK2 V617F negative patients matched by age and gender. Cardiac disorders were characterized as coronary artery disease (CAD), atrial fibrillation (Afib), heart failure (HF), and myocardial infarction (MI). As well as cardiac disorders and systemic hypertension (HTN), we considered the other thrombotic events such as stroke, deep vein thrombosis (DVT), pulmonary embolism (PE) and peripheral arterial disease (PAD). We conclude that there is an association between JAK2 V617F mutation and HTN, CAD, DVT, PAD, HF and Afib.Keywords
Jak2 mutation; Hypertension; Atrial fibrillationAbbreviations
CAD: Coronary Artery Disease; Afib: Atrial Fibrillation; HF: Heart Failure; MI: Myocardial Infarction; HTN: Hypertension; DVT: Deep Vein Thrombosis; PE: Pulmonary Embolism; PAD: Peripheral Arterial Disease; MPNs: Myeloproliferative Neoplasms; WHO: World Health Organization; JAK2: Janus Kinase 2; PV: Polycythemia Vera; PMF: Primary Myelofibrosis; ET: Essential Thrombocythemia; MPL: Myeloproliferative Leukemia Protein; TIA: Transient Ischemic Attack; CVA: Cerebrovascular Accident; MI: Myocardial Infarction; DVT: Deep Venous Thrombosis; MARS: Medical Archival Retrieval SystemIntroduction
Myeloproliferative neoplasms (MPNs) are clonal disorders of hematopoietic stem cells characterized by the proliferation of one or more myeloid lineages. In the revised 2008 World Health Organization (WHO) classification of MPNs, the mutational status of JAK2 (Janus Kinase 2), a tyrosine kinase involved in intracellular signaling, is an essential part of the diagnostic algorithm [1].The JAK2 V617F point mutation has been reported in more than 90% of patients with polycythemia vera (PV) and approximately half of patients with primary myelofibrosis (PMF) and essential thrombocythemia (ET) [2-5]. JAK2 is coupled to several cell-surface hematopoietic growth factor receptors including the EPO receptor and thrombopoietin receptor and MPL (myeloproliferative leukemia protein).
The specific JAK2 point mutation most closely associated with MPNs, causes constitutive activation of the JAK2 kinase domain, which results in erythropoietin- independent erythropoiesis. Approximately 15% of patients with PV suffer either an arterial or, less commonly, a venous thrombotic event within 2 years of diagnosis and 20% with a large vessel thrombotic complication such as a transient ischemic attack (TIA), cerebrovascular accident (CVA), myocardial infarction (MI), deep venous thrombosis (DVT), hepatic vein thrombosis, or thrombosis at other unusual sites.
The specific mechanisms for the predisposition of these patients toward thrombosis remain elusive. Investigators have postulated that increases in blood viscosity, elevations of either the white blood cell or platelet count, increases in platelet reactivity, and/or JAK2 V617F allele burden may play a role. All of these postulations are supported to some degree by both laboratory and clinical corroborative explorations. Effective interventions for JAK2 V617F positive MPNs include phlebotomy, aspirin therapy, cytoreductive therapy, and JAK2 inhibition and all of these interventions are thought to reduce the thrombotic risk associated with MPNs.
Of interest for the retrospective study reported here is the finding by previous investigators that the JAK2 V617F mutation is correlated with both specific cellular activation parameters of the polymorphonuclear leukocytes and platelets and thrombosis in patients with PV and ET. In this regard, reports of JAK2 mutation positive, patients presenting with large vessel venous thrombosis and no clinical evidence of MPN is especially interesting, since this implies that the presence of JAK2 abnormalities alone are of clinical importance and may influence the clinician’s trigger for exploring JAK2 mutation status and the commencement of treatment.
Materials and Methods
We performed a retrospective case series study at our multiinstitutional center. This study was approved by our Institutional review board. We reviewed 1000 patients identified by the hospital Medical Archival Retrieval System (MARS) laboratory data system who had been evaluated for JAK2 V617F mutation from January 2012 - December 2014. Finally, we found 100 JAK2 V617F positive patients and 100 JAK2 V617F negative patients matched by age and gender. We found the frequency of coronary artery disease (CAD), atrial fibrillation (Afib), heart failure (HF), myocardial infarction (MI), stroke, deep vein thrombosis (DVT), pulmonary embolism (PE), peripheral arterial disease (PAD), and hypertension (HTN) in two groups of patients. JAK2 V617F mutation was detected based on allele-specific PCR analysis (Sigma-Proligo and Sigma-Genosys, 3050 Spruce Street, St Louis, MO 63103). CAD and PAD were diagnosed based on angiography reports, Afib and MI were diagnosed based on EKG findings, HF was diagnosed based on echocardiography reports, stroke was diagnosed based on CT-scan reports, and DVT was diagnosed based on Doppler sonography reports. PE was diagnosed based on CT Angiography reports, and HTN was detected in routine check-ups. We collected the demographic information which included age, gender and race. All data were entered into Microsoft excel worksheet and statistical analysis performed using SPSS Version 22. The mean, minimum and maximum range was used to describe non parametric continuous variables such as age. Pearson chi square test was used to assess relationships between groups. Twosided P-value < 0.05 was designated as significant.Results
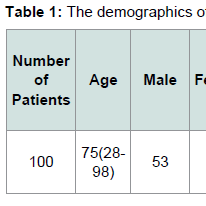
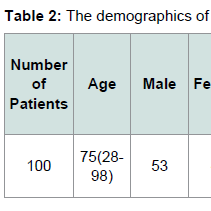
The demographics for our study population are indicated in Tables 1 and 2. Eighty six patients had systemic hypertension. Among these patients 54 patients were JAK2 V617F positive, while 32 patients were JAK2 V617F negative. Sixteen patients had CAD. Among these patients 13 patients were JAK2 V617F positive while 3 patients were JAK2 V617F negative. Twenty two patients had DVT. Among these patients 17 patients were JAK2 V617F positive while 5 patients were JAK2 V617F negative. Eight patients had pulmonary embolism. Among these patients 5 patients were JAK2 V617F positive while 3 patients were JAK2 V617F negative.Sixteen patients had either embolic or ischemic stroke. No patients had hemorrhagic stroke. Among these patients 11 patients were JAK2 V617F positive while 5 patients were JAK2 V617F negative. Eleven Patients had MI. Eight patients were JAK2 V617F positive while 3 patients were JAK2 V617F negative. Fifteen patients had PAD. Among these patients 12 patients were JAK2 V617F positive while 3 patients were JAK2 V617F negative. Fifteen patients had HF. Among these patients 12 patients were JAK2 V617F positive, and 3 patients were JAK2 V617F negative.
Twenty patients had Afib. Fifteen patients were JAK2 V617F positive and 5 patients were JAK2 V617F negative. The frequency of HTN and CAD in JAK2 V617F negative and JAK2 V617F positive patients is shown in Figure 1. The frequency of DVT and stroke in JAK2 V617F negative and JAK2 V617F positive patients is shown in Figure 2. The frequency of MI and PE in JAK2 V617F negative and JAK2 V617F positive patients is shown in Figure 3. The frequency of PAD and HF in JAK2 V617F negative and JAK2 V617F positive patients is shown in Figure 4. The frequency of Afib in JAK2 V617F negative and JAK2 V617F positive patients is shown in Figure 5.
Figure 1: Frequency of HTN and CAD in JAK2 V617F negative and JAK2V617F positive patients.The frequency of HTN in JAK2 V617F positive patients 54% (95% CI: 0.44-0.64) compared to frequency of HTN in JAK2 V617F negative patients 32% (95% CI: 0.23-0.41) was statistically significant P = 0.002, and also the frequency of CAD in JAK2 V617F positive patients 13% (95% CI: 0.06-0.20) compared to frequency of CAD in JAK2 V617F negative patients 3% (95% CI: 0.001-0.06) was statistically significant P = 0.009.
Figure 2: Frequency of DVT and stroke in JAK2 V617F negative and JAK2V617F positive patients.The frequency of DVT in JAK2 V617F positive patients 17% (95% CI: 0.10-0.24) compared to frequency of DVT in JAK2 V617F negative patients 5% (95% CI: 0.01-0.09) was statistically significant P = 0.0007, but the frequency of stroke in JAK2 V617F positive patients 11% (95% CI: 0.05-0.17) compared to frequency of stroke in JAK2 V617F negative patients 5% (95% CI: 0.01-0.09) was not statistically significant P = 0.118.
Figure 3: Frequency of MI and PE in JAK2 V617F negative and JAK2 V617Fpositive patients.The frequency of MI in JAK2 V617F positive patients 8% (95% CI: 0.03-0.13) compared to frequency of MI in JAK2 V617F negative patients 3% (95% CI: 0.001- 0.06) was not statistically significant P = 0.121, and also the frequency of PE in JAK2 V617F positive patients 5% (95% CI: 0.01-0.09) compared to frequency of PE in JAK2 V617F negative patients 3% (95% CI: 0.001-0.06) was not statistically significant P = 0.47.
Figure 4: Frequency of PAD and HF in JAK2 V617F negative and JAK2V617F positive patients.The frequency of PAD in JAK2 V617F positive patients 12% (95% CI: 0.06-0.18) compared to frequency of PAD in JAK2 V617F negative patients 3% (95% CI: 0.001-0.06) was statistically significant P = 0.016, and also frequency of HF in JAK2 V617F positive patients 12% (95% CI: 0.06-0.18) compared to frequency of HF in JAK2 V617F negative patient 3% (95% CI: 0.001-0.06) was statistically significant P = 0.016.
Discussion
Previously published data regarding the association of JAK2V617F mutation status and risk of cardiovascular events are difficult to reconcile. This may be because the prevalence of the JAK2 V617F mutation is low in coronary patients as well as in healthy subjects. Muendlein et al. found no difference in the prevalence of JAK V617F mutation among coronary patients and healthy subjects, making the detection of an association between JAK2 V617F positivity and an increased risk of atherothrombotic complications problematic [6]. In contrast, Gasser et al. demonstrated that the JAK2 V617F mutation is common in coronary patients [7].Furthermore, Muendlein et al. have reported that the frequency of JAK2 V617F positivity among patients with PAD is 5-fold greater than among healthy individuals; a difference that is highly statistically significant (p < 0.001) [8].
JAK2 V617F is infrequently associated with ischemic stroke in the absence of overt MPN [9]. These clinical observations in humans is supported by the demonstration of an association between the JAK2 V617F mutation and cardiac disorders in JAK2 V617F transgenic mice that develop increased ventricular wall thickness, enlarged cardiomyocytes, extensive myocardial inflammatory cell infiltration and collagen fibrosis, and coronary artery thrombosis [10].
Along these lines, investigators have shown that patients with ET and polycythemia vera and increases of their JAK2 V617F allele burden have both an increase in their thrombotic risk , but also demonstrable changes in their prothrombotic status as determined by “ex vivo laboratory investigation. For example, patients with ET have increased levels of platelet-neutrophil aggregates and monocyte CD11b (a cluster of differentiation antigen also referred to as MAC-1) expression were associated with thrombosis supporting the role of leukocyte activation in the pathophysiology of thrombosis [11].
Our study has several strengths and weaknesses. The strength is that the large number of patient records reviewed and included in this report reduces the play of selection bias and our use of age-gender matching techniques reduced the confounding due to these factors. The Weaknesses in our study include the retrospective design which necessitated our reliance on the electronic medical record for patient selection.
We conclude that there is an increased frequency of Afib, stroke, DVT, CAD, HTN, PE, PAD, HF, and MI in patients who are JAK2 V617F positive compared to the patients who are JAK2 V617F negative, However we could only observe a significant statistical association between JAK2 V617F mutation and Afib, CAD, HTN, DVT, PAD, and HF.
Acknowledgements
Hereby I acknowledge Dr. Roy E Smith, Dr. Siddharth Sheth, and Melissa Forkey who helped me to finalize this project.References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. (2008) WHO classification of tumours of haematopoietic and lymphoid tissues, fourth edition. IARC.
- Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, et al. (2005) The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both "atypical" myeloproliferative disorders and myelodysplastic syndromes. Blood 106: 1207-1209.
- Tefferi A, Gilliland DG (2005) The JAK2V617F tyrosine kinase mutation in myeloproliferative disorders: status report and immediate implications for disease classification and diagnosis. Mayo Clin Proc 80: 947-958.
- Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, et al. (2005) Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 106: 2162-2168.
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Eng J Med 352: 1779-1790.
- Muendlein A, Gasser K, Kinz E, Stark N, Leiherer A, et al. (2014) Evaluation of the prevalence and prospective clinical impact of the JAK2 V617F mutation in coronary patients. Am J Hematol 89: 295-301.
- Gasser K, Muendlein A, Stark N, Winder T, Rein P, et al. (2011) Evaluation of the JAK2 V617F mutational status in coronary patients. ASCO Annual Meeting, Chicago, IL: J Clin Oncol 29 Suppl: 6585.
- Muendlein A, Kinz E, Gasser K, Leiherer A, Rein P, et al. (2015) Occurrence of the JAK2 V617F mutation in patients with peripheral arterial disease. Am J Hematol 90: E17-E21.
- Pardanani A, Lasho TL, Morice WG, Pruthi RK, Tefferi A (2007) JAK2V617F is infrequently associated with arterial stroke in the absence of overt myeloproliferative disorder. J Thromb Haemost 5: 1784-1785.
- Shi K, Zhao W, Chen Y, Ho WT, Yang P, et al. (2014) Cardiac hypertrophy associated with myeloproliferative neoplasms in JAK2V617F transgenic mice. J Hematol Oncol 7: 25.
- Coucelo M, Caetano G, Sevivas T, Almeida Santos S, Fidalgo T, et al. (2014) JAK2V617F allele burden is associated with thrombotic mechanisms activation in polycythemia vera and essential thrombocythemia patients. Int J Hematol 99: 32-40.