Journal of Geriatrics and Palliative Care
Download PDF
Research Article
*Address for Correspondence: Laurie Archbald-Pannone, PO Box 801379, Charlottesville, VA 22908, Tel: 434-924-5242; Fax: 434-924-0075; E-mail: la2e@virginia.edu
Citation: Archbald-Pannone LR. Quantitative Fecal Lactoferrin as a Biomarker for Severe Clostridium difficile Infection in Hospitalized Patients. J Geriatrics Palliative Care 2014;2(1): 3.
Copyright © 2014 Lim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Geriatrics and Palliative Care | ISSN 2373-1133 | Volume: 2, Issue: 1
Submission: 11 January 2014| Accepted: 12 March 2014 | Published: 17 March 2014
Quantitative Fecal Lactoferrinas a Biomarker for Severe Clostridium difficile Infection in Hospitalized Patients
Laurie R Archbald-Pannone
- Department of Internal Medicine, University of Virginia, Charlottesville, USA
*Address for Correspondence: Laurie Archbald-Pannone, PO Box 801379, Charlottesville, VA 22908, Tel: 434-924-5242; Fax: 434-924-0075; E-mail: la2e@virginia.edu
Citation: Archbald-Pannone LR. Quantitative Fecal Lactoferrin as a Biomarker for Severe Clostridium difficile Infection in Hospitalized Patients. J Geriatrics Palliative Care 2014;2(1): 3.
Copyright © 2014 Lim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Geriatrics and Palliative Care | ISSN 2373-1133 | Volume: 2, Issue: 1
Submission: 11 January 2014| Accepted: 12 March 2014 | Published: 17 March 2014
Abstract
Background: The incidence and severity of Clostridium difficile infection (CDI) have increased over the past decade, especially among hospitalized patients. In this study, we determined the value of published criteria for severe CDI in predicting 3 month mortality, as well as the utility of fecal lactoferrin as a biomarker for severe CDI.Methods: Pilot Year 1 of IRB approved (HSR-IRB# 13630) prospective cohort study of hospitalized patients with CDI at US academic medical center (10/08-4/10). Medical records of hospitalized patients with clinically diagnosed CDI, via toxin assay, were evaluated to objectively define severe CDI based on current guidelines. A stool sample from CDI diagnosis was analyzed for amount of fecal lactoferrin (IBD-SCAN, TechLab, Inc.). Data was analyzed using SPSS for student’s t-test and chi-squared, significance p ≤ 0.05.
Results: 79 subjects consented and enrolled, mean age was 64 years (standard deviation, sd, 17.2), 48 (61%) female, and average Charlson co-morbidity score was 5.8 (sd 3.8). Subjects with severe CDI were 5 times more likely to die within 3 months of diagnosis (Odds Ratio 5.66 (95% Confidence Interval 2.03-15.79), p=0.001) and had significantly more fecal lactoferrin (580.0 (sd 989.0) vs. 181.7 (sd 244.2) μg/mL, p=0.018), compared to those that did not meet severe CDI criteria.
Conclusion:In this pilot study, subjects who meet defined criteria for severe CDI had higher mortality and more intestinal inflammation. These preliminary results were, however, underpowered to show a direct association of lactoferrin with mortality. Larger cohort studies are needed to optimize a criterion for severe CDI and evaluate a direct association of lactoferrin and mortality in hospitalized patients with CDI.
Keywords
Clostridium difficile infection; Quantitative fecal lactoferrin; Fecal biomarker; Nosocomial infectionsIntroduction
Clostridium difficile (C. difficile) is an anaerobic bacterium that causes a toxin mediated inflammatory diarrhea. The incidence and severity of C. difficile infections (CDI) has been increasing over the past decades. CDI can range from a self-limited diarrheal episode to pseudomembranous colitis and death. The reported mortality rate attributable to CDI has been as high as 16.7% in epidemic settings [1-3]. With innovative treatments such as fecal microbiota transplant and minimally invasive surgical intervention as potential options, the identification of patients at highest risk of poor outcome early in their clinical course is increasingly critical [4-7]. Currently, there is no clinical scale at diagnosis that has been shown to predict a patient’s mortality risk following CDI [8-11]. The current CDI treatment guidelines based treated on defined clinical criteria as published by Zar, et al. [9,12], however, the validity of these criteria has not been established. Additionally, fecal biomarkers of inflammation, such as fecal lactoferrin, have been used inflammatory bowel disease to provide an objective assessment of the intestinal inflammatory burden and disease severity [13-16]. Similarly, fecal lactoferrinmay be a useful biomarker in predicting severity of intestinal inflammation in CDI [17-20]. In this study, we evaluated the current clinical definition of severe CDI in a cohort of hospitalized patients for predicting 3 month mortality. We also determined the associated of lactoferrin with severe CDI.Materials and Methods
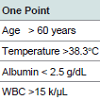
As pilot study, we consented and enrolled a cohort of patients diagnosed with CDI (IRB #13607) during their hospitalization at a University-based tertiary care hospital (October 2008-April 2010). Patients were eligible if they were ≥ 18 years old, diagnosed with CDI during hospitalization with stool sample of adequate volume for analysis, and had no history of chronic diarrhea. The stool sample from CDI diagnoses was collected by clinical microbiology laboratory staff and frozen for analysis. Each associated patient was identified and evaluated by the research team and informed consent was obtained. Subjects were contacted via telephone and medical record was reviewed at 1 and 3 months after diagnosis to determine mortality. All cases of death underwent blinded reviewed by physician to determine cause of death based on detailed review of medical record. Fecal lactoferrin concentration was determined using IBD-SCAN (TechLab, Inc., Blacksburg, VA). Elevated level of fecal lactoferrin was defined as ≥ 7.25 μg/mL [16,19]. A frozen fecal specimen from enrolled subjects on day of CDI diagnosis was shipped to TechLab, Inc., Blacksburg, VA for C. difficile isolation and PCR ribotyping [20].Severe CDI was defined using criteria shown in Table 1 [9,12]. Values recorded for temperature and peripheral white blood cell count were the highest, while values for serum albumin were the lowest recorded within 24 hours of CDI diagnosis. We modified the published criteria so that treatment in Intensive Care Unit (ICU) was defined as ICU care due to CDI (after CDI diagnosis with documentation of diarrhea, dehydration, hypovolemia, acute renal failure, or CDI sepsis in the medical record). Subjects with a score of ≥ 2 points were categorized as having severe CDI. SPSS (v. 20, Chicago, IL) was used for t-test and chi-squared analysis. Statistical significance was set at p ≤ 0.05.
Results
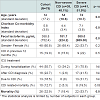
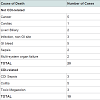
From October 2008- April 2010, 79 hospitalized subjects with CDI were consented and enrolled in this pilot study. Based on the criteria defined, over half of our cohort had severe CDI (n=41, 51.9%, Table 2). Subjects with severe CDI had more comorbidities than the subjects with non-severe CDI. Our cohort had a staggering 38% (n=30) 3 month mortality, with 10 of these deaths potentially related to CDI ( Table 3). Subjects with severe CDI had five-time risk of dying within 3 months (All-cause mortality Odds Ratio (OR) 5.66 (95% Confidence Interval (CI) 2.03-15.79), p=0.001; CDI-related mortality OR 5.06 (95% CI 1.02-25.19), p=0.049), compared to subjects with non-severe CDI. Although the average level of fecal lactoferrin for the cohort was significantly elevated (388.8 (sd 752.4) μg/mL), subjects with severe CDI had significantly higher levels of lactoferrin when compared to those with non-severe CDI (580.0 (sd 989.0) vs. 181.7 (sd 244.2) μg/mL, p=0.018). In this cohort, we could not, however, determine a direct association between lactoferrin and mortality (p=0.787) or ICU treatment (p=0.506). C. difficile was isolated from 19 (24%) of the stool specimen and ribotype 027 was the most prevalent strain isolated (n=7). There was no significant association found between strain of C. difficile isolated and severe CDI. In evaluating the validity of this definition of severe CDI, we found these criteria to have 76.6% sensitivity and 63.3% specificity for predicting 3 month mortality following CDI.Discussion
We evaluated the applicability of a published severity scale in our pilot cohort of hospitalized patients diagnosed with CDI. We found that patients categorized as having severe CDI were older, had more co-morbidity, and were more likely to die within 3 month of the CDI diagnosis. Despite our small sample, we were able to show that subjects with severe CDI had significantly higher degree of intestinal inflammation, as measured by quantitative fecal lactoferrin. When combined with clinical judgment, quantitative fecal lactoferrin could be a useful clinical tool to identify at diagnosis patients who more likely to have a poor outcome and, therefore, may need more aggressive initial treatment.Lactoferrin is a protein that is released from neutrophils and epithelial cells undergoing apoptosis. Lactoferrin has been shown to be a stable and accurate biomarker of leukocyte degranulation seen in intestinal inflammation [16,17,21,22]. Fecal lactoferrin has been used a measure of disease severity in inflammatory bowel disease [4-7]. There have also been published reports demonstrating a correlation with fecal lactoferrin and severe CDI [17-20]. However, there is no consensus as to how best to define severe CDI. In this study, we used the criteria in the current CDI treatment guidelines to define severe CDI [9,12]. However, these criteria did not optimize sensitivity (76.6%) or specificity (63.3%) for predicting mortality in our cohort. Therefore, further studies are needed to define a set of clinical factors with improved ability for classifying and predicting poor outcome for patients diagnosed with CDI during a hospitalization.
A limitation of our study is also our small cohort. This report represents a pilot study for which we will enroll a cohort of 350 subjects over 2 years. We will apply similar analysis to determine significant differences between subjects with poor outcomes from CDI and illuminate important factors at diagnosis. The goal of the on-going study is to develop a tool that provides clinicians with early identification of patients at highest risk for poor outcome following CDI.
Summary
There is no objective measure to predict which patients will have poor outcome following hospitalization with Clostridium difficile infection (CDI). Using established criteria for severe CDI in a pilot study, we show that patients with severe CDI have higher quantitative fecal lactoferrin. Quantitative lactoferrin could be a biomarker for severe CDI and should be evaluated as a potential objective predictor of poor outcome in hospitalized patients.Acknowledgements
This study was funded through NIH/NIAID Grant: 1K23AI074681. All stools were collected by UVA Clinical Microbiology laboratory and received by our research laboratory technician, Charlotte Martin. Special thanks to Jesus E Sevilleja and Poonum Korpe, for assistance with laboratory methods for quantitative fecal lactoferrin.References
- Warny M, Pepin J, Fang A, Kilgore G, Thompson A, et al. (2005) Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366: 1079-1084.
- Pepin J, Valiquette L, Cossette B (2005) Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 173: 1037-1042.
- Pepin J, Valiquette L, Gagnon S, Routhier S, Brazeau I (2007) Outcomes Of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol 102: 2781-2788.
- Kassam Z, Lee C, Yua, Y, Hunt R (2013) Fecal Microbiota Transplantation for Clostridium difficile Infection: Systematic Review and Meta-Analysis. Am J Gastroenterol 108: 500-508.
- Debast SB, Bauer MP, Kuijper EJ, Committee (2014) European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2: 1-26.
- Neal M, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS (2011) Diverting Loop Ileostomy and Colonic Lavage: An Alternative to Total Abdominal Colectomy for the Treatment of Severe, Complicated Clostridium difficile Associated Disease. Ann Surg 254: 423-427.
- Causey MW, Walker A, Cummings M, Johnson EK, Maykel JA, et al. (2014) Colonic decompression and direct intraluminal medical therapy for Clostridium difficile-associated megacolon using a tube placed endoscopically in the proximal colon. Colorectal Dis 16: O71-74.
- Huggan P, Murdoch D (2007) Vancomycin therapy for severe Clostridium difficile–Associated diarrhea. Clin Infect Dis 45: 1647-1648.
- Zar F, Bakkanagari S, Moorthi K, Davis M (2007) A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–Associated diarrhea, stratified by disease severity. Clini Infect Dis 45: 302-307.
- Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, et al. (2007) Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect 55: 495-501.
- Fujitani S, George WL, Murthy AR (2011) Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol 32: 220-228.
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, et al. (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31: 431-455.
- Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, et al. (2003) Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol 98: 1309-1314.
- Walker TR, Land ML, Kartashov A, Saslowsky TM, Lyerly DM, et al. (2007) Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 44: 414-422.
- Yamamoto T, Shiraki M, Bamba T, Umegae S, Matsumoto K (2013) Fecal calprotectin and lactoferrin as predictors of relapse in patients with quiescent ulcerative colitis during maintenance therapy. Int J Colorectal Dis.
- Kopylov U, Rosenfeld G, Bressler B, Seidman E (2014) Clinical Utility of Fecal Biomarkers for the Diagnosis and Management of Inflammatory Bowel Disease. Inflamm Bowel Dis.
- Steiner TS, Flores CA, Pizarro TT, Guerrant RL (1997) Fecal lactoferrin, interleukin-1beta, and interleukin-8 are elevated in patients with severe Clostridium difficile colitis. Clin Diagn Lab Immunol 4: 719-722.
- LaSala PR, Ekhmimi T, Hill AK, Farooqi I, Perrotta PL (2013) Quantitative fecal lactoferrin in toxin-positive and toxin-negative Clostridium difficile specimens. J Clin Microbiol 51: 311-313.
- Boone JH, DiPersio JR, Tan MJ, Salstrom SJ, Wickham KN, et al. (2013) Elevated lactoferrin is associated with moderate to severe Clostridium difficile disease, stool toxin, and 027 infection. Eur J Clin Microbiol Infect Dis 32: 1517-1523.
- Boone JH, Archbald-Pannone LR, Wickham KN, Carman RJ, Guerrant RL, et al. (2014) Ribotype 027 Clostridium difficile infections with measurable stool toxin have increased lactoferrin and are associated with a higher mortality. Eur J Clin Microbiol Infect Dis.
- Sugi K, Saitoh O, Hirata I, Katsu K (1996) Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol 91: 927-934.
- Foell D, Wittkowski H, Roth J (2009) Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 58: 859-868.