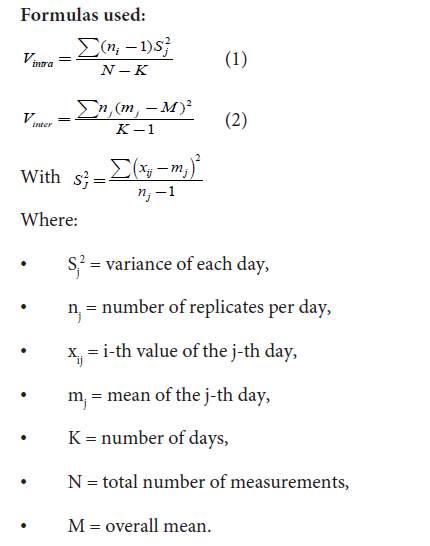Journal of Food Processing & Beverages
Download PDF
The diluted distillate, along with alcoholometers and a 500 mL graduated cylinder, was placed in an incubator maintained at 20°C for one hour to allow thermal equilibration. Following incubation, the equipment and distillate were transferred to an air-conditioned room at 20°C for measurement.
• C is the methanol concentration in the beverage (mg/L),
• q is the daily consumption volume (L/day),
Pc is the conventional adult body weight (70 kg), as defined by the WHO and FAO Environmental Health Criteria 240 (World Health Organization & Food and Agriculture Organization of the United Nations, 2009). [10]
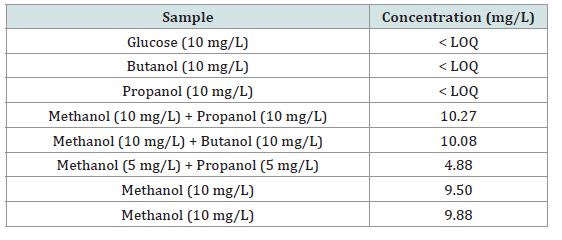
The specificity of the developed method was assessed by testing compounds likely to be present in alcoholic beverages, including glucose, butanol, and propanol, each at 10 mg/L. As presented in [Table 1], none of these compounds produced a signal above the limit of quantification (LOQ), indicating that they do not interfere with methanol detection. In contrast, samples containing methanol, whether alone or mixed with propanol or butanol, showed measured concentrations close to the expected values (9.50–10.27 mg/L for 10 mg/L methanol solutions and 4.88 mg/L for 5 mg/L methanol solutions). These results confirm that the method specifically detects methanol without significant cross-reactivity from other alcohols or hydroxylated compounds, thus ensuring the reliability of measurements in complex beverage matrices.
Research Article
Assessment of Methanol Levels and Labeling Irregularities in Alcoholic Beverages from Yaounde Markets
Songue SO1*, Ekani V2, Tiendo PS1, Mbassi JEG3 and Sado S2
1Hygiene and Environment Department, Physocochemistry Section,
Centre Pasteur du Cameroun
2University of Yaounde 1, Department of biochemistry, Cameroon.
3Institute of Agricultural Research for Development (IRAD), Cameroon.
2University of Yaounde 1, Department of biochemistry, Cameroon.
3Institute of Agricultural Research for Development (IRAD), Cameroon.
*Address for Correspondence:SONGUE SAME Olivier, Hygiene and Environment department,
physicochemical section, Centre Pasteur du Cameroun (CPC). E-mail Id: songueolivier@gmail.com
Submission: 15 August 2025
Accepted: 09 September 2025
Published: 12 September 2025
Accepted: 09 September 2025
Published: 12 September 2025
Copyright: © 2025 Songue SO, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Keywords:Methanol; Alcoholic Beverages; Public Health; Food
Safety; Labeling Compliance
Abstract
This study investigates methanol contamination and labeling
compliance in alcoholic beverages marketed in Yaoundé, Cameroon.
A total of 106 beverages, including spirits, wines, and traditional
drinks, were analyzed. Methanol quantification was performed
using a modified chromotropic acid spectrophotometric method,
while alcohol content was determined by distillation followed by
aerometry. Results revealed that 32.1% of beverages exceeded the
European Union’s methanol safety limit of 50 mg/L, although none
reached the acute toxicity threshold of 2000 mg/L (14 mg/kg bw/day).
Labeling analysis showed that 13.5% of samples had alcohol content
discrepancies, and 16% lacked proper alcohol labeling, particularly
among traditional beverages. Additionally, major traceability gaps,
such as missing or repeated batch numbers, were observed. While
acute methanol poisoning risk appears low, the potential long-term
health impacts of chronic low-level exposure remain concerning,
especially for heavy consumers. The findings highlight the urgent
need for national methanol regulations, stricter labeling enforcement,
systematic beverage monitoring, and public awareness initiatives to
ensure consumer safety and support public health policy development
in Cameroon.
Introduction
The celebration of both joyful and somber events is often
accompanied by the consumption of alcoholic beverages such as
beer, wine, or spirits. Additionally, some individuals consume alcohol
recreationally or as a means of escaping reality, potentially leading
to dependence or addiction (Institut National de la Santé et de la
Recherche Médicale, 2023). [1] In 2016, alcohol consumption ranked
as the seventh leading cause of global mortality, accounting for more
than three million deaths (Matene Fongang, 2020) [2]. Cameroon is
recognized as the largest consumer of alcohol in Central and West
Africa. In 2016 alone, Cameroonians consumed over 660 million liters
of beer (Matene Fongang, 2020) [2]. Globally, more than a quarter of
alcohol consumption is unrecorded, illicit, or undeclared (Manning
& Kowalska, 2021; Probst et al., 2018) [3,4]. In developing countries
such as Cameroon, in addition to industrially produced or imported
beverages, traditional alcoholic drinks are commonly available; these
are often produced with limited mastery of manufacturing practices
(Kubo et al., 2014) [5].
Illicit, adulterated, and poor-quality alcoholic products pose
serious risks to public health and safety (Manning & Kowalska, 2021)
[3]. The lack of strict control over the production process can lead to
contamination by harmful substances such as methanol. Methanol
(CH₃OH) is a volatile, flammable primary alcohol characterized by a
slightly sweet taste and an odor similar to that of ethanol. Methanol
in fermented and alcoholic beverages may have a natural origin,
resulting from specific fermentation techniques (Aït Daoud et al.,
2021; Destanoğlu & Ateş, 2019; Hodson et al., 2017; Ohimain, 2016;
Tomsia et al., 2022) [6-9]. However, cases of intentional methanol
addition to beverages have been reported, primarily aimed at
artificially increasing alcohol content and reducing production
costs, often targeting financially vulnerable consumers [7] (Hodson
et al., 2017; World Health Organization & Food and Agriculture
Organization of the United Nations, 2009) [7,10]. Mass poisonings by
methanol-containing liquids have been reported from Russia (Jargin,
2017) [11].
Clinical manifestations of methanol intoxication range
from symptoms of drunkenness, gastrointestinal disorders,
ocular complications (e.g., optic neuritis), to metabolic and
neuropsychiatric disturbances, which may resolve spontaneously
within a few hours to days post-ingestion (Sanaei-Zadeh, 2012)
[12]. In severe cases, symptoms such as mild mydriasis, bilaterally
non-reactive pupils, and profound metabolic acidosis can occur.
Methanol ingestion represents a major health hazard due to its
potential to cause irreversible organ damage or death if not treated
promptly (Ohimain, 2016; Sanaei-Zadeh, 2012; Tomsia et al.,
2022). [8,12,9] In light of these health risks, various countries have
established regulatory limits for methanol concentrations in alcoholic
beverages. Since 2008, the European Union (EU) has set maximum
allowable methanol concentrations (per liter of pure ethanol) at
13.5 g/L for fruit brandies, 10 g/L for pomace brandies, and 2 g/L
for citrus brandies (Botelho et al., 2020) [13]. In the United States,
Australia, and New Zealand, the limit for spirits and fruit brandies is
7 g/L (Botelho et al., 2020) [13]. Regarding wines, the International
Organization of Vine and Wine (OIV) recommends maximum
methanol concentrations of 250 mg/L for white and rosé wines,
and 400 mg/L for red wines (Thanasi et al., 2024) [14]. The OIV
also mandates specific labeling requirements for wines and spirits,
including the product name, actual alcohol content, batch number,
and responsible producer’s identification (Thanasi et al., 2024) [14].
Physicochemical analyses are essential to ensure both the
accuracy of labeled alcohol content and the absence of harmful
levels of contaminants such as methanol. Several analytical methods
have been developed for methanol detection, including Fourier
Transform Infrared Spectroscopy (Sharma et al., 2009) [15], Raman
Spectroscopy (Boyaci et al., 2012), [16] enzymatic assays (Kučera
& Sedláček, 2017), [17] electrochemical sensors (Kavita et al.,
2022), liquid chromatography (Albaseer & Dören, 2022) [18], gas
chromatography (Sharma et al., 2009; Zamani et al., 2019) [15,19],
and spectrophotometry (Ghadirzadeh et al., 2019; Zamani et al.,
2019) [20,19]. Currently, gas chromatography is recognized as the
gold-standard method for the determination of volatile alcohols in
beverages according to European Commission Regulation (EC) No.
2870/2000, due to its specificity and high sensitivity (Zamani et al.,
2019). [19] However, its high operational cost, the requirement for
rare gases, and the need for highly specialized personnel limit its
routine application in developing countries.
Spectrophotometry using chromotropic acid has been proposed
by the OIV as a low-cost alternative for methanol analysis in wines and
spirits, although the method remains a Type IV analytical method. A
Type IV Method or Tentative Method is a method which has been
used traditionally or else has been recently introduced but for which
the criteria required for acceptance by the Codex Committee on
Methods of Analysis and Sampling have not yet been determined
(Codex Alimentarius, 2019). [21] Recent studies have enhanced this
method, demonstrating acceptable sensitivity and quantification
limits when compared to gas chromatography (Ghadirzadeh et al.,
2019; Zamani et al., 2019). [20,19]
The present study aims to assess the exposure risk to methanol
among populations in Yaoundé through the consumption of
alcoholic beverages. This represents the first investigation of its kind
in Cameroon and establishes a foundation for broader research on
methanol contamination in the country. The findings also advocate
for the implementation of systematic regulatory controls using cost effective
analytical kits.
Methodology
Reagents:
The following reagents were used in this study: chromotropic
acid, ethanol (100%), methanol (99.9%), and sodium metabisulfite
(98%) were purchased from VWR Chemicals (Europe, France).
Phosphoric acid (85%), potassium permanganate (>99.5%), and
sulfuric acid (98%) were obtained from MERCK (Europe, Germany).
The prepared solutions were:
- Chromotropic acid solution (0.05% in 75% sulfuric acid v/v):
Dissolve 50 mg of chromotropic acid (or its sodium salt) in35 mL of distilled water. Cool the solution in an ice bath, then
carefully add 75 mL of concentrated sulfuric acid (density
1.84 g/mL) in small portions while stirring continuously.
- Standard methanol solution: 0.5 g/L methanol prepared in
5% (v/v) ethanol.
- Dilution solution: 50 mL of absolute ethanol diluted to 1 L
with distilled water.
Equipment:
The actual alcohol content of the beverages was determined using
a RAYPA distiller (ENODES, Spain). All weighings were performed
with an Adventurer SL analytical balance (OHAUS, Switzerland)
with a precision of four decimal places. Alcohol content of the
distillates was measured using a series of alcoholometers (ranges
0–10, 20–30, 30–40, and 40–50) supplied by ALLA (ALLA-France,
France). Methanol quantification was carried out with a Jenway 7205
UV-Visible spectrophotometer (Cole-Parmer Ltd, UK) following its
reaction with chromotropic acid. Samples and analytical equipment
were stored at 20°C in a Bio Expert refrigerated incubator (Froilabo,
France).Samples:
Samples of wines, spirits, and traditional beverages were collected
from July to November 2018 and from June to October 2023 at the
Mokolo Market (GPS: 3.8742049 N, 11.502111 E) in Yaoundé, the
capital city of Cameroon. Mokolo Market is one of the largest and
most populous marketplaces in Central Africa. Beverage brands
were selected based on a preliminary survey conducted with
market vendors to identify the most frequently consumed products.
A total of 106 samples were purchased, representing approximately
80% of the most popular brands across wines, spirits, and traditional
beverages. For each brand, three bottles were acquired. The batch
number, labeled alcohol content, nominal volume, manufacturer
information, and production and expiration dates were recorded
for each sample. To maintain confidentiality, brand names are not
disclosed in this study.Specifically, 60 spirit brands, 36 wine brands, and 10 types of
traditional beverages were sampled. The products were transported
at ambient temperature in containers shielded from direct sunlight
and placed in secure areas of the transport vehicle to prevent physical
damage. Upon arrival, samples were stored in air-conditioned rooms
maintained at 20°C to minimize methanol volatility at elevated
temperatures (>60°C).
Determination of the Actual Alcohol Content of Beverages:
The actual alcohol content of the beverages was determined using
a distillation followed by aerometry method. In this procedure, 200
mL of each beverage sample was distilled using the RAYPA distiller.
The distillate was collected in a 500 mL volumetric flask and diluted
to the mark with ultra-pure water.The diluted distillate, along with alcoholometers and a 500 mL graduated cylinder, was placed in an incubator maintained at 20°C for one hour to allow thermal equilibration. Following incubation, the equipment and distillate were transferred to an air-conditioned room at 20°C for measurement.
The distillate was carefully poured into the graduated cylinder,
and an alcoholometer was immersed into the liquid. Once the
alcoholometer stabilized, standing perpendicular to the surface, the
reading at the liquid interface was recorded. The recorded value was
multiplied by 2.5 to calculate the actual alcohol content of the original
beverage. The selection of the alcoholometer was based on the
expected alcohol content: the first alcoholometer used corresponded
to a range approximately 2.5 times lower than the labeled alcohol
percentage. If the alcoholometer was completely submerged, a lower range
instrument was selected; conversely, if it floated excessively with
no readable graduation at the surface, a higher-range alcoholometer
was employed.
Each measurement was performed in duplicate to ensure
reliability. Based on the determined alcohol content, the distillate
was further diluted as needed to reach an approximate 5% alcohol
concentration for subsequent analytical procedures.
Methanol Quantification in Beverages:
The quantification of methanol was based on its oxidation to
formaldehyde by potassium permanganate acidified with phosphoric
acid, using beverage distillates diluted to 5% (v/v) alcohol. The
resulting formaldehyde reacts with chromotropic acid to form a
purple-colored complex, which absorbs maximally at 575 nm. The
absorbance intensity is proportional to the methanol concentration,
as measured by UV-Visible spectrophotometry.The method applied in this study is a modification of the
procedure described by Ghadirzadeh et al., (2019). [20] The principal
modification consists of introducing an ice water bath step prior
to the addition of saturated potassium permanganate to prevent
formaldehyde volatilization and ensure greater reaction stability.
Calibration standards were prepared by diluting 100% methanol
into 5% (v/v) ethanol to achieve a stock solution of 500 mg/L
methanol. Serial dilutions were then performed by introducing 2.5,
5, 10, 15, 20, and 25 mL of the stock solution into 50 mL volumetric
flasks, corresponding to final methanol concentrations of 25, 50, 100,
150, 200, and 250 mg/L, respectively.
For quantification, 500 μL of either a standard solution or a
beverage distillate adjusted to 5% (v/v) alcohol was transferred into
test tubes. The volume was brought up to the gauge mark with 5%
ethanol (prepared by diluting absolute ethanol with ultra-pure water).
Subsequently, 50 μL of 50% phosphoric acid was added to each tube.
After cooling the tubes in an ice bath, 100 μL of saturated potassium
permanganate solution was added. Following a 10-minute reaction
time, the excess permanganate was decolorized with 100–200 μL of a
2% neutral sodium sulfite solution.
Then, 5 mL of a 0.05% chromotropic acid solution in 98% sulfuric
acid was added to each tube. The tubes were incubated in a water
bath at 70°C for 20 minutes, then cooled to room temperature for 20
minutes before absorbance measurements were performed at 575 nm.
All measurements were conducted in duplicate to ensure analytical
reliability.
Method Validation and Quality Control:
All glassware was washed with a mild detergent, rinsed three
times with tap water, and subsequently rinsed twice with ultra-pure
water prior to use. The linearity of the method was evaluated based
on calibration curves constructed from methanol standard solutions
at concentrations of 25, 50, 100, 150, 200, and 250 mg/L. Regression
lines were generated by plotting concentration against optical density
values obtained after chromotropic acid complexation.Precision was assessed at three concentration levels; each tested
in quadruplicate within a single day to evaluate intra-day variability
(Equation 1). Intermediate (inter-day) precision was determined by
repeating the same spiking tests on two additional, separate days,
allowing calculation of inter-day variance (Equation 2). Both intraday
and inter-day precision were expressed as coefficients of variation
(CV, %).
To verify the absence of contamination during distillation,
blank tests were performed using 5% (v/v) ethanol prepared
with ultra-pure water in place of beverage samples.
Limits of detection (LOD) and quantification (LOQ) were calculated
by analyzing ten blank samples. The LOD was defined as the mean
blank value plus three times the standard deviation (SD), and the
LOQ as the mean plus ten times the SD.
The maximum method bias was determined as the percentage deviation between the mean spiked sample recovery and the expected theoretical value based on the calibration curve.
The maximum method bias was determined as the percentage deviation between the mean spiked sample recovery and the expected theoretical value based on the calibration curve.
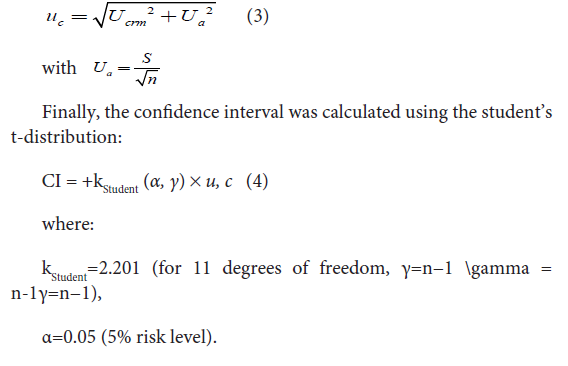
The maximum uncertainty was calculated based on the 95%
confidence interval (CI) of the precision tests. Initially, uncertainty
of certified reference material (ucrm) was read directly on the label of
standard solutions. Then, expanded uncertainty of certified reference
material (Ucrm) was determined by multiplying ucrm by two (Ucrm
= 2 × ucrm). Random uncertainty (Ua) and combined uncertainty
(Uc) were then calculated as follows:
Evaluation of Human Exposure:
Human exposure to methanol through the consumption of
alcoholic beverages was estimated using the following equation:
E=C q
p´
Where :
• E is the exposure value expressed in μg/kg body weight (bw)
per day,• C is the methanol concentration in the beverage (mg/L),
• q is the daily consumption volume (L/day),
Pc is the conventional adult body weight (70 kg), as defined by the WHO and FAO Environmental Health Criteria 240 (World Health Organization & Food and Agriculture Organization of the United Nations, 2009). [10]
Pc is the conventional adult body weight (70 kg), as defined by the
WHO and FAO Environmental Health Criteria 240 (World Health
Organization & Food and Agriculture Organization of the United
Nations, 2009). [10]
The daily consumption value (q) was set at 500 mL (0.5 L) per person, based on data from the National Institute of Statistics (INS) and as reported by Ingenbleek et al., (2017), [21] representing the intake pattern of heavy consumers.
The daily consumption value (q) was set at 500 mL (0.5 L) per person, based on data from the National Institute of Statistics (INS) and as reported by Ingenbleek et al., (2017), [21] representing the intake pattern of heavy consumers.
Data Processing and Statistical Analysis:
Data analysis was performed using Microsoft Excel 2016,
primarily for plotting calibration curves and calculating descriptive
statistics (means, standard deviations, coefficients of variation).
Variance analysis (ANOVA) and box plot visualizations were carried
out using R software, version 4.0.2, to assess differences between
beverage categories and to support statistical interpretations of
methanol concentration distributions.Results and Discussion
Choice of Method:
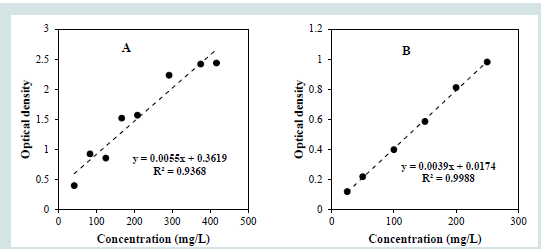
The type IV method described by the International Organization
of Vine and Wine OIV (Codex Alimentarius, 2019; OIV, 2009)
[21,23] was modified by introducing a cooling step prior to the
addition of potassium permanganate to improve method stability
and linearity [Figure 1]. In the conventional method [Figure 1A], the
calibration curve shows moderate linearity (R² = 0.9368), likely due
to the volatility of methanal formed during the reaction, especially
at temperatures above 25°C. This volatility leads to variability in the
amount of methanal available for reaction with chromotropic acid,
compromising reproducibility.After modification, the calibration curve (Figure 1B) exhibits
significantly improved linearity (R² = 0.9988) over the concentration
range of 2.5–250 mg/L. The cooling step minimizes methanal loss
by volatilization, leading to a more stable and reliable analytical
response. Thus, the modified method offers enhanced precision and
accuracy for methanol quantification in alcoholic beverages.
Verification of the Performance of the Chosen Method:
• SpecificityThe specificity of the developed method was assessed by testing compounds likely to be present in alcoholic beverages, including glucose, butanol, and propanol, each at 10 mg/L. As presented in [Table 1], none of these compounds produced a signal above the limit of quantification (LOQ), indicating that they do not interfere with methanol detection. In contrast, samples containing methanol, whether alone or mixed with propanol or butanol, showed measured concentrations close to the expected values (9.50–10.27 mg/L for 10 mg/L methanol solutions and 4.88 mg/L for 5 mg/L methanol solutions). These results confirm that the method specifically detects methanol without significant cross-reactivity from other alcohols or hydroxylated compounds, thus ensuring the reliability of measurements in complex beverage matrices.
Figure 1:Calibration curves for the quantification of methanol using (A) the
conventional OIV method and (B) the modified OIV method.
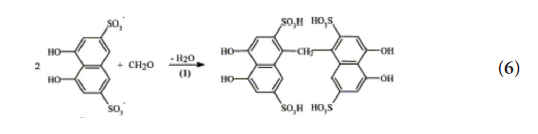
It is evident that chromotropic acid reacts only with methanal
according to Equation (6) described by Fagnani, (2003). [24] It does
not react with other forms of aldehyde.
However, if the drink that is to react with chromotropic acid is
the result of fermentation that induces the production of formic acid
or formaldehyde, the latter will also govern. In this case, we see false
positive results (Zamani et al., 2019) [19].
• Limits of Detection and Quantification:
The tests conducted ten times with the blank (0.5% ethanol
alcoholic solution) allowed for the calculation of the mean and
standard deviation, necessary for determining the limits of
quantification (LOQ) and detection (LOD) (Section 2.6). It appears
that the limits of quantification and detection are 2.68 mg/L and 0.81
mg/L, respectively. Considering the methanol content in beverages
commonly viewed in the literature (>5 mg/L), the quantification and
detection limits of the method are therefore appropriate. They are
comparable to the detection limits presented by (Ellis et al., 2019) [25]
in their work on the quantification of methanol in counterfeit spirits
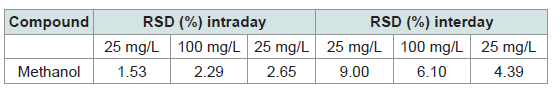
using Raman spectroscopy.• Precision, Uncertainties, and Bias:
The precision of the method was evaluated through intra-day
and inter-day repeatability tests at concentrations of 25 mg/L and
100 mg/L [Table 2]. The intra-day relative standard deviations
(RSD) were 1.53% and 2.29% for 25 mg/L and 100 mg/L methanol
solutions, respectively, while inter-day RSDs were 2.65% and 9.00%.
These values are all below the generally accepted threshold of 10%,
confirming good repeatability and intermediate precision of the
method. The highest variability was observed in the inter-day test
at 100 mg/L, yet it remained within acceptable limits for analytical
methods. Overall, the low RSD values demonstrate that the method
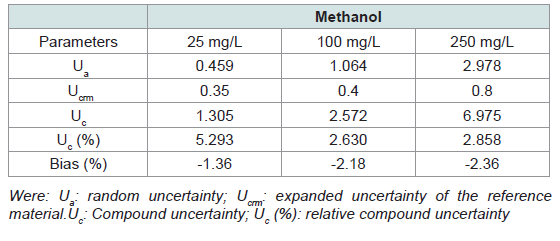
produces reliable and consistent results under the tested conditions.[Table 3] also presents the uncertainties and bias of the
method. Bias and uncertainty tests demonstrated that the methanol
quantification method maintained good analytical performance
across all concentration levels. Relative combined uncertainties
(Uc%) remained below 6%, and biases ranged from –1.36% to
–2.36%, indicating slight but acceptable underestimations. These
results confirm the method’s accuracy and suitability for reliable
methanol analysis in alcoholic beverages.
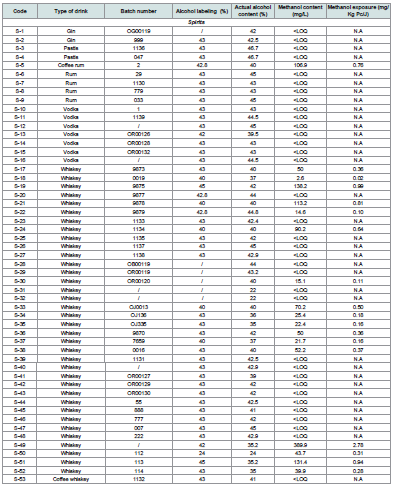
Ethanol and Methanol Content in Beverages: Exposure Evaluation:
The analysis of 106 alcoholic beverages, including 60 spirits, 36
wines, and 10 traditional drinks, revealed considerable variability in
methanol content depending on the beverage type [Table 4]. Among
the spirits, 64.1% of samples had methanol concentrations below
the limit of quantification (LOQ), whereas 35.9% showed detectable
levels, with concentrations reaching up to 138.2 mg/L. Although
these levels remained below the acute toxicity threshold of 2000 mg/L
(Ohimain, 2016),[8] several whiskey samples exceeded the European
Union’s recommended safety limit of 50 mg/L, suggesting potential
risks related to uncontrolled distillation practices or adulteration
(Ellis et al., 2019). [25]Almost half of the red wine samples (47.4%) exhibited methanol
concentrations above 100 mg/L, with the highest recorded value
reaching 206.5 mg/L. Nevertheless, all wine samples complied with
the OIV regulatory limits for methanol content in red (500 mg/L)
and white wines (250 mg/L) (Thanasi et al., 2024). [14] The elevated
methanol levels in wines are likely associated with pectin degradation
during fruit fermentation, a natural source of methanol production
(Md et al., 2013; Navianti et al., 2018). [26,27][28,29]
Traditional beverages, particularly palm wine and odontol,
generally exhibited lower methanol concentrations, ranging from
14.5 to 40.3 mg/L. However, most palm wine samples exceeded limit
fixed by National Agency for Food, Drug Administration and Control
(NAFDAC) (5 mg/L) for traditional alcoholic beverages (Ohimain,
2016). [8] The absence of specific national standards for methanol
content in Cameroon allows such practices to persist, affecting local
consumer safety and complicating exports to neighboring countries
with stricter regulations.
Labeling and traceability assessments also revealed major
deficiencies: 13 beverages (13.5%) showed discrepancies between the
actual and declared alcohol content, while 17 samples (16%), mainly
traditional beverages, lacked any alcohol labeling. Additionally, 15%
of spirits and 39% of wines were missing batch numbers, and some
producers assigned identical batch numbers across different brands,
suggesting possible fraud or at least poor-quality control practices.
Regarding health risk assessment, the estimated methanol
exposure from beverage consumption ranged from 0.02 to 1.48 mg/
kg body weight/day. Although these values remained well below the
lethal exposure threshold of 14 mg/kg body weight/day (Ohimain,
2016) [8], chronic low-dose exposure could pose long-term health
risks, particularly targeting the central nervous system and visual
pathways (Sanaei-Zadeh, 2012) [12].
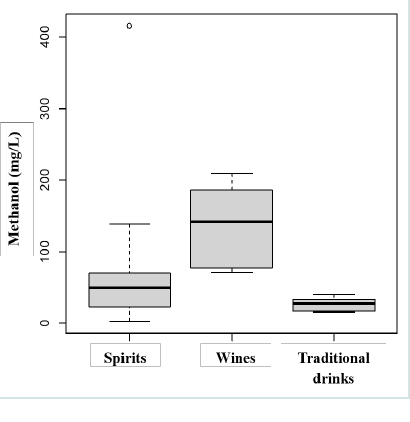
Variance analysis [Figure 2] showed no significant differences
between the methanol contents of spirits and wines (P = 0.126) or
between spirits and traditional beverages (P = 0.099). However, a
significant difference was observed between wines and traditional
beverages (P = 0.0003), reflecting greater variability in spirit
formulations, particularly among whiskeys of various types (e.g.,
fruity, skimmed, coffee-flavored varieties).
Overall, these findings underscore the urgent need for Cameroon
to establish national regulations governing methanol content in both
industrial and traditional alcoholic beverages. In addition, systematic
monitoring, stricter labeling enforcement, and public awareness
initiatives are essential to protect public health. The significant
variability in methanol concentrations, combined with widespread
labeling non-compliance, highlights an urgent call for regulatory
action. The key implications and recommendations arising from this
study are discussed in the following conclusion.
Conclusion
This study provides a comprehensive evaluation of methanol
contamination and labeling compliance in alcoholic beverages sold
in Yaoundé, Cameroon. Results showed that 32.1% of beverages
contained methanol levels exceeding the European Union’s safety
threshold of 50 mg/L. Although none of the samples reached
acute toxicity levels (2000 mg/L or 14 mg/kg/day), the potential
chronic effects of low-dose methanol exposure, particularly among
heavy consumers, remain a significant concern. Moreover, major
labeling deficiencies, including inaccurate alcohol declarations
and missing batch numbers, highlight critical gaps in product
traceability and regulatory oversight. In the absence of national
standards, urgent governmental action is required to establish
methanol limits aligned with international guidelines, enforce
strict labeling practices, and implement systematic quality controls.
Future studies should expand sampling, particularly for locally
produced traditional beverages, to better characterize risks and
support the development of comprehensive public health policies.