Journal of Food Processing & Beverages
Download PDF





Research Article
Vitamin D3 and Consciousness Energy Healing TreatmentModality on Human Osteoblast Cells
Snehasis Jana*, Jay Antony Prague 1,Mahendra Kumar Trivedi 1, Alice Branton 1, Dahryn Trivedi 1, Gopal Nayak 1, Sambhu Charan Mondal2
- 1Trivedi Global Inc., USA
- 2Trivedi Science Research Laboratory Pvt Ltd, Madhya Pradesh,India
*Address for Correspondence: Snehasis Jana, Trivedi Science Research Laboratory Pvt Ltd, Hall-A, Chinar Mega Mall, Chinar Fortune City, Bhopal-462026, Madhya Pradesh, India, Tel: +91-755-6660006; E-mail: publication@trivedisrl.com
Citation: Prague JA, Trivedi MK, Branton A, Trivedi D, Jana S. Vitamin D3 and Consciousness Energy Healing Treatment Modality on Human Osteoblast Cells. J Food Processing & Beverages. 2018;6(1): 7.
Copyright: © 2018 Prague JA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium,provided the original work is properly cited.
J Food Processing & Beverages | ISSN: 2332-4104 | Volume: 6, Issue: 1
Submission: 23 April, 2018| Accepted: 10 May, 2018 | Published: 17 May, 2018
Abstract
The current study was established to evaluate the effect ofConsciousness Energy Healing based vitamin D3 and DMEM medium on bone cells development. The test items were divided into two parts. One part of each sample received Consciousness Energy Healing Treatment by Jay Antony Prague and those samples were labeled as Biofield Treated (BT) samples, while other parts of each sample were denoted as untreated test items (UT). MTT data showed test samples were found as safe in tested concentrations. ALP was significantly increased by 214.74%, 350.53%, and 536.32% in UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 0.1 μg/mL compared to UT-DMEM + UT-Test item group. Further, ALP level was significantly elevated by 155.09% and 93.37% in BT-DMEM + UT-Test item and BT-DMEM + BT-Test item groups, respectively at 1 μg/mL compared to untreated. Collagen was significantly increased by 73.40%, 150.39%, and 133.59% in UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 1 μg/mL compared to untreated. Moreover, level of collagen was significantly increased by 105.68%, and 72.76% in BT-DMEM + UT-Test item and BT-DMEM + BT-Test item groups, respectively at 50 μg/mL with respect to untreated. Besides, percent of bone mineralization was distinctly increased by 229.99%, 229.99%, and 200% in UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BTDMEM + BT-Test item groups, respectively at 0.1μg/mL compared to untreated. Percent of bone mineralization was distinctly increased by 137.27% and 142.17% in UT-DMEM + BT-Test item and BT-DMEM + UT-Test item groups, respectively at 100 μg/mL, while increased by 78.29% and 96.71% in UT-DMEM + BT-Test item and BT-DMEM + UT-Test item groups, respectively as compared to untreated. Overall, Biofield energized vitamin D3 has a significant effect on bone health and able to combat vitamin D3 deficiency and fight against various bone-related problems.
Keywords
Vitamin D; The Trivedi Effect®, Biofield Energy HealingTreatment; Osteosarcoma cells (MG-63); Bone development
Abbreviations
MG-63: Human Bone Osteosarcoma Cells; ALP: AlkalinePhosphatase; CAM: Complementary and Alternative Medicine; NHIS: National Health Interview Survey; NCCIH: National Center of Complementary and Integrative Health; DMEM: Dulbecco’s Modified Eagle’s Medium; FBS: Fetal Bovine serum; ATCC: American Type Culture Collection; UT: Untreated; BT: Biofield Energy Treated; TI: Test Item
Introduction
Vitamin D has multiple effects, which regulate the functions in different organs viz. brain, liver, lungs, heart, kidneys, skeletal, immune and reproductive systems. Moreover, it has significant antiinflammatory, anti-aging, anti-stress, anti-arthritic, anti-osteoporosis, anti-apoptotic, wound healing, anti-cancer, anti-psychotic and antifibrotic actions [1]. Vitamin D receptors are widely distributed in most of the body organs viz. brain, liver, heart, lungs, kidney, pancreas, large and small intestines, muscles, reproductive, nervous system, etc. Vitamin D receptors influence cell-to-cell communication, normal cell growth, cell differentiation, cell cycling and proliferation, hormonal balance, neurotransmission process, skin health, immune and cardiovascular functions. In any living vertebrates, vitamin D plays an important role in maintaining a healthy skeletal structure and is essential for bone health. Naturally, it is synthesized in the presence of sunlight in the skin [2]. Most foods do not contain any vitamin D, additionally nowadays due to aging, use of sunscreen, and change of zenith angle of sun the production of vitamin D3 has reduced [3]. Increasing age is not only related to a decrease in bone marrow depression and muscle strength but is also associated with marked changes in the immune and inflammatory responses [4]. Deficiency of vitamin D3 causes metabolic bone diseases like osteomalacia and exacerbate osteoporosis, etc [5]. The quality of life for meno pausal women is one of the most critical health problems in the today world. Metabolic bone disorders like osteoporosis are mainly prevalent in post-menopausal women. Hormonal factors and rapid bone loss in post-menopausal women leads to an increased risk of fractures [6]. Hence, the serum calcium and alkaline phosphatase (ALP) levels in post-menopausal women are the main two vital biochemical markers of bone metabolism. However, bone-specific ALP is the most important marker for osteoblast differentiation [7]. Further, it is generally accepted that an increased calcium intake along with an adequate source of vitamin D is important for maintaining good bone health. Vitamin D also plays an important role in maintaining an adequate level of serum calcium and phosphorus. Therefore, vitamin D has a great impact in forming and maintaining strong bones [8,9]. Bone strength depends on the quality, geometry, shape, micro architecture, turnover, mineral content, and the collagen content. Collagen is the major structural protein responsible for bone calcification. In the aging state, the mechanical properties of the bones become impaired and the bones get fragile, that causes various clinical disorders associated with bone collagen abnormalities and bone fragilities, such as Osteogenesis imperfecta and osteoporosis [10,11].
In recent years, several scientific reports and clinical trials have revealed the useful effects of Biofield Energy Treatments, which have shown to enhance immune function in cases of cervical cancer patients via therapeutic touch [12], massage therapy [13], etc. Complementary and Alternative Medicine (CAM) therapies are now rising as preferred models of treatment, among which Biofield Therapy (or Healing Modalities) is one approach that has been reported to have several benefits to enhance physical, mental and emotional human wellness. However, as per the data of 2012 from the National Health Interview Survey (NHIS), which indicated that the highest percentage (17.7%) of the Americans used dietary supplements as a complementary health approach as compared with other practices in past years. The National Center of Complementary and Integrative Health (NCCIH) has recognized and accepted Biofield Energy Healing as a CAM health care approach in addition to other therapies, medicines and practices such as natural products, deep breathing, yoga, Tai Chi, Qi Gong, chiropractic/osteopathic manipulation, meditation, massage, special diets, homeopathy, progressive relaxation, guided imagery, acupressure, acupuncture, relaxation techniques, hypnotherapy, healing touch, movement therapy, pilates, rolfing structural integration, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines, naturopathy, essential oils, aromatherapy, Reiki, and cranial sacral therapy. Human Biofield Energy has subtle energy that has the capacity to work in an effective manner [14]. CAM therapies have been practiced worldwide with reported clinical benefits in different health disease profiles [15]. This energy can be harnessed and transmitted by the experts into living and non-living things via the process of Biofield Energy Healing. Biofield Energy Treatment (The Trivedi Effect®) has been published in numerous peer-reviewed science journals with significant outcomes in many scientific fields such as cancer research, microbiology, biotechnology, pharmaceutical science, agricultural science, materials science, nutraceuticals, skin health, human health and wellness [16-37].
Based on the literature information and importance of vitamin D3 on bone health, the authors sought to evaluate the impact of the Biofield Energy Treatment (The Trivedi Effect®) on the test samples (vitamin D3 and DMEM medium) for bone health activity with respect to the assessment of different bone health parameters like ALP, collagen content, and bone mineralization using standard assays in MG-63 cells.
Materials and Methods
Chemicals and reagents
Fetal Bovine Serum (FBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Life Technology, USA. Rutin hydrate was purchased from TCI, Japan, while vitamin D3 (denoted as test item) and L-ascorbic acid were obtained from Sigma-Aldrich, USA. Antibiotic solution (penicillin-streptomycin) was procured from HiMedia, India, while 3-(4, 5-dimethyl-2- thiazolyl)-2, 5-diphenyl-2H-tetrazolium) (MTT), Direct Red 80, and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma, USA. All the other chemicals used in this experiment were analytical grade, which procured from India.
Cell culture
The human bone osteosarcoma cell line, MG-63, was used as the test system in the present study. The MG-63 cell linewas maintained under the DMEM growth medium for routine culture and supplemented with 10% FBS. Growth conditions were maintained at 37 °C, 5% CO2 and 95% humidity and sub cultured by trypsinisation followed by splitting the cell suspension into fresh flasks and supplementing with fresh cell growth medium. Three days before the start of the experiment (i.e. day -3), the growth medium of near-confluent cells was replaced with fresh phenol-free DMEM, supplemented with 10% charcoal dextran stripped FBS (CD-FBS) and 1% penicillin-streptomycin [38].
Experimental design
The experimental groups consisted of untreated cells group (baseline control), vehicle control groups (0.05% DMSO with Biofield Energy Treated and untreated DMEM), a positive control group (rutin hydrate) and experimental test groups. Experimental groups included the combination of the Biofield Energy Treated and untreated vitamin D3/DMEM. It consisted of four major treatment groups on specified cells with UT-DMEM + UT-Test item, UT-DMEM + Biofield Energy Treated test item (BT-Test Item), BT-DMEM + UT-Test Item, and BTDMEM + BT-Test Item.
Consciousness energy healing treatment strategies
The test item (vitamin D3) and DMEM were divided into two parts. One part each of the test item and DMEM were treated with the Biofield Energy (also known as The Trivedi Effect®) and coded as the Biofield Energy Treated items, while the second part did not receive any sort of treatment and was defined as the untreated samples. This Biofield Energy Healing Treatment was provided by Jay Antony Prague, who participated in this study and performed the Biofield Energy Treatment remotely for ~5 minutes. Jay Antony Prague was remotely located in the USA, while the test samples were located in the research laboratory of Dabur Research Foundation, New Delhi, India. The Biofield Energy Treatment was administered for 5 minutes through the healer’s unique Energy Transmission process remotely to the test samples under laboratory conditions. Jay Antony Prague in this study never visited the laboratory in person, nor had any contact with the test item and medium. Further, the control group was treated with a sham healer for comparative purposes. The sham healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy Treated and untreated samples were kept in similar sealed conditions for experimental study.
Determination of non-cytotoxic concentration
The cell viability test was performed by MTT assay in the human bone osteosarcoma cell line (MG-63). The cells were counted and plated in 96-well plates at the density corresponding to 5 X 103 to 10 X 103 cells/well/180 μL of cell growth medium. The above cells were incubated overnight under growth conditions and allowed cell recovery and exponential growth, and then they were subjected to serum stripping or starvation. The cells were treated with the test item, DMEM, and the positive control. The untreated cells served as the baseline control. The cells in the above plate(s) were incubated for a time point ranging from 24 to 72 hours in a CO2 incubator at 37 °C, 5% CO2and 95% humidity. Following incubation, the plates were taken out and 20 μL of 5 mg/mL of MTT solution was added to all the wells followed by an additional incubation for 3 hours at 37 °C. The supernatant was aspirated and 150 μL of DMSO and was added to each well to dissolve formazan crystals. The absorbance of each well was read at 540 nm using a Synergy HT microplate reader, BioTek, USA. The percentage cytotoxicity at each tested concentration of the test substance was calculated using the following Equation 1:

Where, X = Absorbance of treated cells; R = Absorbance of untreated cells
The percentage cell viability corresponding to each treatment was then be obtained using the following Equation 2:

The concentrations exhibiting ≥70% Cell viability was considered as non-cytotoxic [39].
Assessment of alkaline phosphatase (ALP) activity
The cells were counted using a hemocytometer and plated in a 24- well plate at the density corresponding 1 x 104 cells/well in phenol-free DMEM supplemented with 10 % CD-FBS. Following the respective treatments, the cells in the above plate were incubated for 48 hours in a CO2 incubator at 37 °C, 5% CO2 and 95% humidity. After 48 hours of incubation, the plate was taken out and processed for the measurement of ALP enzyme activity. The cells were washed with 1XPBS and lysed by freeze-thaw method i.e., incubation at -80 °C for 20 minutes followed by incubation at 37 °C for 10 minutes. To the lysed cells, 50 μL of substrate solution i.e. 5 mM of p-nitrophenyl phosphate (pNPP) in 1M diethanolamine and 0.24 mM magnesium chloride (MgCl2) solution (pH 10.4) was added to all the wells followed by incubation for 1 hour at 37 °C. The absorbance of the above solution was read at 405 nm using Synergy HT microplate reader (Biotek, USA). The absorbance values obtained were normalized with substrate blank (pNPP solution alone) absorbance values. The percentage increase in ALP enzyme activity with respect to the untreated cells (baseline group) was calculated using Equation 3:

Where, X = Absorbance of cells corresponding to positive control and test groups
R = Absorbance of cells corresponding to baseline group (untreated cells).
Assessment of collagen synthesis
The MG-63 cells were counted using a hemocytometer and plated in 24-well plate at the density corresponding to 10 x 103 cells/well in phenol-free DMEM supplemented with 10% CD-FBS. Following the respective treatments, the cells in the above plate were incubated for 48 hours in a CO2 incubator at 37 °C, 5% CO2 and 95% humidity. After 48 hours of incubation, the plate was taken out and the amount of collagen accumulated in MG-63 cells corresponding to each treatment was measured by Direct Sirius red dye binding assay. In brief, the cell layers were washed with PBS and fixed in Bouin’s solution (5% acetic acid, 9% formaldehyde and 0.9% picric acid) for 1 hour at room temperature (RT). After 1 hour of incubation, the above wells were washed with milliQ water and air dried. The cells were then stained with Sirius red dye solution for 1 hour at RT followed by washing in 0.01 N HCl to remove unbound dye. The collagen dyecomplex obtained in the above step was dissolved in 0.1 N NaOH and absorbance was read at 540 nm using Biotek Synergy HT microplate reader. The level of collagen was extrapolated using standard curve obtained from purified Calf Collagen Bornstein and Traub Type I (Sigma Type III). The percentage increase in collagen level with respect to the untreated cells (baseline group) was calculated using Equation 4:

Where, X = Collagen levels in cells corresponding to positive control and test groups
R = Collagen levels in cells corresponding to baseline group (untreated cells).
Assessment of bone mineralization by alizarin red S staining
The MG-63 cells were counted using a hemocytometer and plated in 24-well plate at the density corresponding to 10 x103 cells/well in phenol-free DMEM supplemented with 10% CD-FBS. Following the respective treatments, the cells in the above plate were incubated for 48 hours in a CO2 incubator at 37 °C, 5% CO2 and 95% humidity to allow cell recovery and exponential growth. Following overnight incubation, the above cells were subjected to serum stripping for 24 hours. The cells were then treated with non-cytotoxic concentrations of the test samples and positive control. Following 3-7 days of incubation with the test samples and positive control, the plates were taken out, cell layers processed further by staining with Alizarin Red S dye. The cells were fixed in 70% ethanol for 1 hour, after which Alizarin Red solution (40 μm; pH 4.2) was added to the samples for 20 minutes with shaking. The cells were washed with distilled water to remove unbound dye. For quantitative analysis by absorbance evaluation, nodules were solubilized with 10% cetylpyridinium chloride for 15 minutes with shaking. Absorbance was measured at 562 nm using Biotek Synergy HT microplate reader. The percentage increase in bone mineralization with respect to the untreated cells (baseline group) was calculated using the following Equation 5:

Where, X = Absorbance in cells corresponding to positiv control or test groups
R = Absorbance in cells corresponding to baseline (untreated) group.
Statistical analysis
All the values were represented as percentage of the respective parameters. For statistical analysis Sigma-Plot (version 11.0) was used as a statistical tool. Statistically significant values were set at the level of p≤0.05.
Results and Discussion
MTT assay
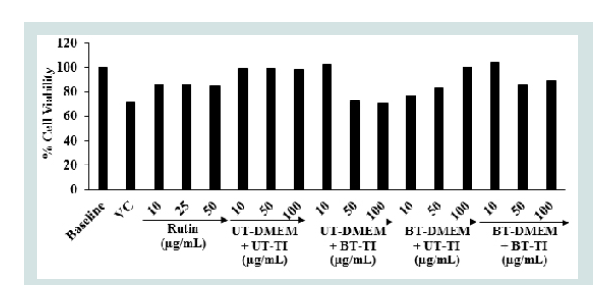
The results of the cell viability data after treatment with the Biofield Energy Treated test items in MG-63 cells are depicted in Figure 1. The data revealed that the test items did not exhibit any cytotoxicity (as evidence of cell viability approximately greater than 70%) across all the tested concentrations up to 100 μg/mL. Hence, the same concentrations were assessed further to see the effect of the test samples on the levels of alkaline phosphatase (ALP) activity, collagen synthesis, and bone mineralization in MG-63 cells.
Alkaline phosphatase (ALP) activity
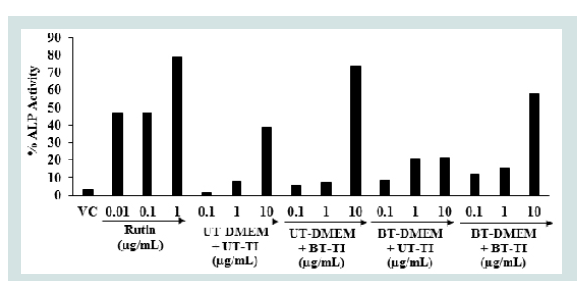
The effect of the test items on alkaline phosphatase (ALP) enzyme in human bone osteosarcoma cells is shown in Figure 2. The level of ALP was observed 3.3% in the vehicle control (VC) group compared to the untreated cells group. The ALP activity was significantly increased by 46.62%, 47.37%, and 78.95% in the positive control (rutin) group at the concentration of 0.01, 0.1, and 1 μg/mL, respectively compared to the untreated cells group. The level of ALP was increased by 214.74%, 350.53%, and 536.32% in the UT-DMEM + BT-Test item and BTDMEM + UT-Test item, and BT-DMEM + BT-Test item groups, at the concentration of 0.1 μg/mL compared to the UT-DMEM + UTTest item group. Further, the level of ALP was significantly increased by 155.09% and 93.37% in the BT-DMEM + UT-Test item and BTDMEM + BT-Test item groups, respectively at 1 μg/mL compared to the UT-DMEM + UT-Test item group. Additionally, the level of ALP was significantly increased by 90.88% and 50.54% in the UTDMEM + BT-Test item and BT-DMEM + BT-Test item groups, respectively at 10 μg/mL compared to the UT-DMEM + UT-Test item group. Overall, the Consciousness Energy Healing Treated (The Trivedi Effect®) test item group (i.e. vitamin D3) showed an improved synthesis of ALP level in the human osteosarcoma cells with respect to the untreated item items group. Vitamin D is essential for the maintenance of skeletal health [40]. ALP is an essential biomarker for the diagnosis of vitamin D deficiency [41]. Therefore, the deficiency of vitamin D affect’s on various tissues. For physiological bone growth contributes to elevated level of serum alkaline phosphatase activity and, hence, the level of serum ALP activity is 1.5 to 2.5 times higher in growing children than in normal adults [42]. On the contrary, decreased alkaline phosphatase activity has been observed in cases such as cessation of bone growth, achondroplasia, and cretinism [43]. In this experiment, it was also evident that the Biofield Energy Treated Vitamin D3 significantly increased the level of ALP expression, which might be very advantageous to maintain a healthy skeletal structure for the patients suffering from various bone related disorders.
Figure 1: Representation of the cell viability of the test items (vitamin D3 andDMEM medium) in different concentrations in MG-63 cells after 72 hours oftreatment.
Assessment of collagen activity
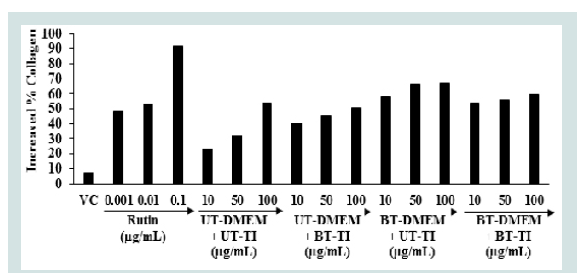
The effect of the Biofield Energy Treated test samples on collagen synthesis in human bone osteosarcoma cells is shown in Figure 3. Collagen level in the VC group was observed as 7.2% as compared to the untreated cells group. The level of collagen synthesis was significantly elevated by 48.35%, 52.96%, and 92.2% at 0.001, 0.01, and 0.1 μg/mL, respectively in the positive control group compared to the untreated cells group. The collagen synthesis was significantly increased by 73.40%, 150.39%, and 133.59% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 10μg/mL compared to the UT-DMEM + UT-Test item group. Moreover, the collagen level was significantly increased by 41.14%, 105.68%, and 72.76% in the UT-DMEM + BTTest item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 50μg/mL compared to the UT-DMEM + UTTest item group. Additionally, at 100μg/mL the level of collagen was also significantly increased by 23.86% and 9.85% in the BT-DMEM + UT-Test item and BT-DMEM + BT-Test item groups, respectively with respect to the UT-DMEM + UT-Test item group (Figure 3).The literature showed that the active metabolite i.e., 1,25-(OH)2D3 of vitamin D which regulates the synthesis of collagen by changing a translational efficiency as well as mRNA abundance [44]. It also increased the level of the bone-specific calcium-binding protein, osteocalcin by approximately 20 folds, which ultimately stimulates a partial differentiation to the osteoblast phenotype in MG-63 cells [45]. Overall, The Trivedi Effect® - Consciousness Energy Healing Treatment modality showed a significant improvement of the collagen synthesis in human osteosarcoma cells. Thus, it is assumed that The Trivedi Effect® has the potential to improve the bone cells differentiation and development in various skeletal disorders.
Assessment of bone mineralization by alizarin red S (ARS) staining
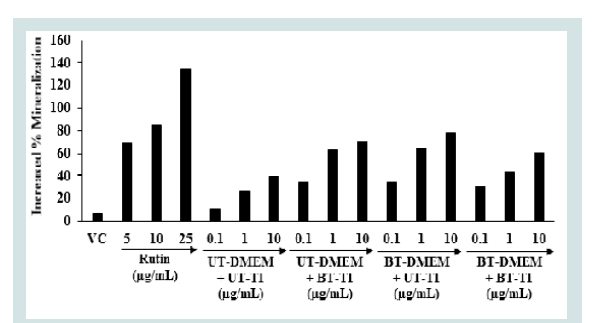
The Alizarin red S (ARS) staining is widely utilized for the assessment of calcium-rich deposits in the cell culture study [46]. Vitamin D3 is important for calcium and phosphate homeostasis [47]. The active metabolite of vitamin D3 indirectly influenced the stimulation of calcium uptake, re-absorption, and modifies various osteoblast differentiation and mineralization-related genes, such as alkaline phosphatase (ALPL), osteocalcin (BGLAP), and osteopontin (SPP1) in human osteoblasts in vitro [48]. The bone mineralization expressed as % in human bone osteosarcoma cells is shown in Figure 4. The positive control rutin showed 68.93%, 84.60%, and 134.46% increased the percentage of bone mineralization at 5, 10, and 25 μg/ mL, respectively as compared to the untreated cells group. The results of the Biofield Energy Treated test items showed that the percent of bone mineralization was significantly raised by 229.99%, 229.99%, and 200% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 0.1 μg/ mL compared to the UT-DMEM + UT-Test item group. Further, a significant increased the percentage of bone mineralization by 137.27%, 142.17%, and 64.72% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 1 μg/mL with respect to the UT-DMEM + UT-Test item group. In addition, the data showed a significant increased of bone mineralization by 78.29%, 96.71%, and 51.97% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively than the UT-DMEM + UT-Test item group (Figure 4) at 10 μg/mL. Thus, based on the above findings it showed that the Consciousness Energy Healing Treatment (The Trivedi Effect®) based test item groups (i.e. vitamin D3) showed a remarkable improvement of bone mineralization content assessed by in vitro in the human osteosarcoma cells (MG-63) with respect to the all others treatment groups.
Conclusion
For the assessment of cytotoxic nature of the test items, the MTT cell viability assay was performed and the data showed greater than 70% cells were viable, which indicated that the test samples were safe and nontoxic in the tested concentrations upto 100 μg/mL. ALP was significantly increased by 214.74%, 350.53%, and 536.32% in the UTDMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 0.1 μg/mL; while increased by 155.09% and 93.37% in the BT-DMEM + UT-Test item and BTDMEM + BT-Test item group at 1 μg/mL compared to the UT-DMEM + UT-Test item group. Collagen was significantly increased by 73.40%, 150.39%, and 133.59% UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 1μg/mL with respect to the untreated group. Additionally, the level of collagen was significantly increased by 105.68%, and 72.76% in the BT-DMEM + UT-Test item and BT-DMEM + BT-Test item groups, respectively at 50 μg/mL with respect to the untreated group. Besides, the percent of bone mineralization was distinctly increased by 229.99%, 229.99%, and 200% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 0.1μg/mL compared to the untreated group. The percent of bone mineralization was distinctly increased by 137.27% and 142.17% in the UT-DMEM + BT-Test item and BT-DMEM + UT-Test item groups, respectively at 100 μg/mL, while increased by 78.29% and 96.71% in the UT-DMEM + BT-Test item and BT-DMEM + UT-Test item groups, respectively as compared to the untreated group. Altogether, the Biofield Energy Treated test samples (The Trivedi Effect®) demonstrated a significant impact on bone health parameters. Therefore, the Consciousness Energy Healing based vitamin D3 might be suitable for the development of an alternative and more effective supplement for vitamin D3 deficiency, which could be useful for the management of various bone-related disorders viz. low bone density and osteoporosis, osteogenesis imperfecta, Paget’s disease of bone, rickets, osteomalacia, bone and joint pain, bone fractures, deformed bones, osteoma, chondrodystrophia fetalis, etc. Besides, it can also be utilized in organ transplants (for example kidney transplants, liver transplants and heart transplants), various autoimmune disorders such as Lupus, Addison Disease, Celiac Disease (gluten-sensitive enteropathy), Dermatomyositis, Graves’ Disease, Hashimoto Thyroiditis, Multiple Sclerosis, Myasthenia Gravis, Pernicious Anemia, Aplastic Anemia, Reactive Arthritis, Rheumatoid Arthritis, Sjogren Syndrome, Systemic Lupus Erythematosus, Type 1 Diabetes, Alopecia Areata, Crohn’s Disease, Fibromyalgia, Vitiligo, Psoriasis, Scleroderma, Chronic Fatigue Syndrome and Vasculitis, as well as inflammatory disorders such as Asthma, Ulcerative Colitis, Alzheimer’s Disease, Atherosclerosis, Dermatitis, Diverticulitis, Hepatitis, Irritable Bowel Syndrome, inflammatory diseases, antiinflammatory, anti-stress, anti-arthritic, anti-osteoporosis, antiapoptotic, wound healing, anti-cancer, anti-psychotic and antifibrotic actions stress management and prevention, and anti-aging by improving overall health, Parkinson’s Disease and stress etc. To modulate the immune system by improving overall health.
Figure 4: The effect of the test samples on human bone osteosarcoma cellsfor the assessment of bone mineralization activity.
References
- Holick MF (2004) Sunlight and vitamin D for bone health and prevention ofautoimmune diseases cancers, and cardiovascular disease. Am J Clin Nut 80(6 Suppl): 1678S-1688S.
- Holick MF (1996) Vitamin D and bone health. J Nutr 126 (4 Suppl): 1159S-1164S.
- Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF et al. (1987) Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab 64: 1165-1168.
- Barnes MS, Robson JP, Bonham MP, Strain JJ, Wallace JMW et al. (2006) Vitamin D: Status, supplementation and immunodulation. Cur Nut Food Sci 2: 315-336.
- Laird E, Ward M, McSorley E, Strain JJ, Wallace J et al. (2010) Vitamin D and bone health; Potential mechanisms. Nutrients 2: 693-724.
- Bhattarai T, Bhattacharya K, Chaudhuri P, Sengupta P (2014) Correlation of common biochemical markers for bone turnover, serum calcium, and alkaline phosphatase in post-menopausal women. Malays J Med Sci 21: 58-61.
- Iba K, Takada J, Yamashita T (2004) The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab 22: 594-596.
- Holick MF, Garabedian M (2006) Vitamin D: Photobiology, metabolism, mechanism of action, and clinical applications. Primer on the metabolic bone diseases and disorders of mineral metabolism. Edited by: Favus MJ, Washington, DC.
- DeLuca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80 (6 Suppl): 1689S-1696S.
- Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17: 319-336.
- Sroga GE, Vashishth D (2012) Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 10: 141-150.
- Lutgendorf SK, Mullen-Houser E, Russell D, Degeest K, Jacobson G, et al. (2010) Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun 24: 1231-1240.
- Krishnamoorthy S, Kunjithapatham S, Manickam L (2013) Traditional Indian breakfast (Idli and Dosa) with enhanced nutritional content using millets. Nutr Diet pp: 241–246.
- Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R, et al. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. Glob Adv Health Med 4 (Suppl): 58-66.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8: 703-717.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S et al. (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S et al. (2015) Invitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S et al. (2015) Invitro evaluation of biofield treatment on Enterobacter cloacae: Impact on antimicrobial susceptibility and biotype. J Bacteriol Parasitol 6: 241.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S et al. (2015) Evaluation of biofield modality on viral load of hepatitis B and C Viruses. J Antivir Antiretrovir 7: 83-88.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S et al. (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis 5: 189.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, Jana S et al. (2015) Antimicrobial sensitivity, biochemical characteristics and biotyping of Staphylococcus saprophyticus: An impact of biofield energy treatment. J Women’s Health Care 4: 271.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Jana S et al. (2015) Evaluation of antibiogram, genotype and phylogenetic analysis of biofield treated Nocardia otitidis. Biol Syst Open Access 4: 143.
- Supavititpatana P, Wirjantoro TI, Raviyan P (2010)Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S et al. (2015) Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield. Pharm Anal Acta 6:395.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S et al. (2015) Spectroscopic characterization of biofield treated metronidazole and tinidazole. Med Chem 5: 340-344.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S, et al. (2015) Effect of biofield treatment on spectral properties of paracetamol and piroxicam. Chem Sci J 6: 98.
- Trivedi MK, Branton A, Trivedi D, Shettigar H, Bairwa K, et al. (2015) Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. Nat Prod Chem Res 3: 186.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2016) Molecular analysis of biofield treated eggplant and watermelon crops. Adv Crop Sci Tech 4: 208.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango pp: 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of plant growth, yield and yield attributes of biofield energy treated mustard and chick pea seeds 4: 291-295.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of plant growth regulator, immunity and DNA fingerprinting of biofield energy treated mustard seeds 4: 269-274.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Characterization of physical and structural properties of aluminum carbide powder: Impact of biofield treatment. J Aeronaut Aerospace Eng 4: 142.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Impact of biofield treatment on atomic and structural characteristics of barium titanate powder. Ind Eng Manage 4: 166.
- Trivedi MK, Patil S, Nayak G, Jana S, Latiyal O (2015) Influence of biofield treatment on physical, structural and spectral properties of boron nitride. J Material Sci Eng 4: 181.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Characterization of physical and structural properties of brass powder after biofield treatment. J Powder Metall Min 4: 134.
- Mahendra KT, Gopal N, Shrikant P, Rama MT, Snehasis J et al. (2015) Bio-field treatment: An effective strategy to improve the quality of beef extract and meat infusion powder. J Nutr Food Sci 5: 389.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Biofield treatment: A potential strategy for modification of physical and thermal properties of gluten hydrolysate and ipomoea macroelements. J Nutr Food Sci 5: 414.
- Czekanska EM, Stoddart MJ, Richards RG, Hayes JS (2012) In search of an osteoblast cell model for invitro research. Eur Cell Mater 24: 1-17.
- Biological evaluation of medical devices - Part 5: Tests for invitro cytotoxicity (ISO 10993-5:2009), I.S.EN ISO, 10993-10995
- Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266-281.
- Matsuo K, Mukai T, Furuya A, Suzuki S, Tanahashi Y, et al. (2013) A case of vitamin D deficiency without elevation of serum alkaline phosphatase in a carrier of hypophosphatasia. Clin Pediatr Endocrinol 22: 73-76.
- Masrour Roudsari J, Mahjoub S (2012) Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and paget’s specimens. Caspian J Intern Med 3:478-483.
- Kennel KA, Drake MT, Hurley DL (2010) Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin Proc 85: 752-758.
- Franceschis RT, Romano PR, Parks KY(1989) Regulation of type I collagen synthesis by 1,25-Dihydroxyvitamin D3 in human osteosarcoma cells. J Biol Chem 263: 18938-19945.
- Kurihara N, Ishizuka S, Kiyoki M, Haketa Y, Ikeda K et al. (1986) Effects of 1,25-dihydroxyvitamin D3 on osteoblastic MC3T3-E1 cells. Endocrinology118: 940-947.
- Yusa K, Yamamoto O, Takano H, Fukuda M, Iino M et al. (2016) Zinc-modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro. Sci Rep 6: 29462.
- Shaker JL, Deftos L, De Groot LJ, Chrousos G, Dungan K et al.(2018) Calcium and phosphate homeostasis. South Dartmouth (MA): MDText.com, Inc.
- van de Peppel J, Johannes PTM, van Leeuwen (2014) Vitamin D and gene networks in human osteoblasts. Front Physiol 5: 137.