Journal of Food Processing & Beverages
Download PDF
Research Article
*Address for Correspondence: Tapani Alatossava, Department of Food and Environmental Sciences, P.O. Box 66, FI-00014 University of Helsinki, Helsinki, Finland, Tel: +358 9191 58312; Fax: +358 9 191 58460; E-mail: tapani.alatossava@helsinki.fi
Citation: Alatossava T, Li R, Munsch-Alatossava P. From “Viili” Towards “Termoviili”, a Novel Type of Fermented Milk: Characterization of Growth Conditions and Factors for a Co-culture of Lactobacillus delbrueckii and Geotrichum candidum. J Food Processing & Beverages. 2013;1(2): 8.
Copyright © 2013 Alatossava T et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 1, Issue: 2
Submission: 11 November 2013 | Accepted: 12 December 2013 |Published: 18 December 2013
Bacteriological Media, Milk Bases and Chemicals
Microorganisms, their Culture, and their Enumeration Procedures
Synergistic properties of the co-culture of Lactobacillus delbrueckii ssp. lactis ATCC 15808 and Geotrichum candidum VV in various milk bases
Formic acid, a Growth Factor of Lactobacillus delbrueckii ssp. lactis strain ATCC 15808
In principle, the citrate catabolism based diacetyl flavor production by mesophilic LAB in “Viili” is replaced by yogurt aroma (mainly acetaldehyde) production by Lb. delbrueckii in “Termoviili”. The slight sharpness of the flavor may be due to CO2 production or consequent to lipolysis by G. candidum. The characteristic high viscosity property of “Viili” is based on the presence of ropy LAB (especially L. lactis ssp. cremoris) starter strains responsible for EPS (slime) production. Hence by applying EPS producing Lb. delbrueckii starter strain(s) and, if required, additional adjunct culture(s), the desired texture and flavour properties of “Termoviili” could be modified and optimized. As compared to yogurt, the presence of G. candidum could improve the nutritional properties of “Termoviili” through the consumption of dissolved O2 (anti-oxidative environment) and through the synthesis of some vitamins and other valuable bioactive compounds by G. candidum.
From “Viili” Towards “Termoviili”, a Novel Type of Fermented Milk: Characterization of Growth Conditions and Factors for a Co-culture of Lactobacillus delbrueckii and Geotrichum candidum
Tapani Alatossava*, Ruojie Li and Patricia Munsch-Alatossava
- Department of Food and Environmental Sciences, University of Helsinki, Finland
*Address for Correspondence: Tapani Alatossava, Department of Food and Environmental Sciences, P.O. Box 66, FI-00014 University of Helsinki, Helsinki, Finland, Tel: +358 9191 58312; Fax: +358 9 191 58460; E-mail: tapani.alatossava@helsinki.fi
Citation: Alatossava T, Li R, Munsch-Alatossava P. From “Viili” Towards “Termoviili”, a Novel Type of Fermented Milk: Characterization of Growth Conditions and Factors for a Co-culture of Lactobacillus delbrueckii and Geotrichum candidum. J Food Processing & Beverages. 2013;1(2): 8.
Copyright © 2013 Alatossava T et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 1, Issue: 2
Submission: 11 November 2013 | Accepted: 12 December 2013 |Published: 18 December 2013
Abstract
The traditional Northern fermented milk product “Viili” is based on the use of a starter comprising both mesophilic lactic acid bacteria (LAB) and Geotricum candidum mold strains for milk fermentation at 18 to 20°C for about 20 hours. The goal of the present study was to investigate whether there is a microbiological basis for the conception of a novel type of fermented milk product “Termoviili”, which would be a hybrid between “Viili” and yogurt, because of the use of thermophilic Lactobacillus delbrueckii instead of mesophilic LAB as a starter component. Accordingly some critical growing conditions and factors that support a co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV strains were determined and further compared to a yogurt starter like co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and Streptococcus thermophilus T101 strains. Both Lb. delbrueckii and G. candidum could grow at a temperature range of 30-34°C, and optimally at 30°C. At this temperature, the co-culture of Lb. delbrueckii and G. candidum showed similar synergistic interactions, like the yogurt starter co-culture, when growth occurred in a milk base, which had not been subjected to strong heat treatment like autoclaving at 121°C for 10 minutes. Formic acid, at a concentration of 7.35 mM, stimulated the growth of Lb. delbrueckii ssp. lactis ATCC 15808, likewise when G. candidum VV or Str. themophilus T101 were grown in a coculture with Lb. delbrueckii ssp. lactis ATCC 15808. These results suggest that G. candidum, like Str. thermophilus, produces formic acid which stimulates the growth of Lb. delbrueckii. Furthermore, a strong heat treatment of the milk base generated the same stimulatory effect on Lb. delbrueckii, as observed when Lb. delbrueckii was grown in the presence of 7.35 mM formate. The results obtained demonstrate that a co-culture of Lb. delbrueckii and G. candidum is based on a synergistic association, and accordingly these two species could be applied together as a starter in the manufacture of “Termoviili”, a novel type of fermented milk product with potential new sensory and nutritional properties.Keywords
Viili; Yoghurt; Lactobacillus delbrueckii; Streptococcus thermophilus; Geotrichum candidum; Formic acid; Milk heat treatmentIntroduction
Fermented milks have been manufactured and consumed for a long time in human history. Various starters including lactic acid bacteria (LAB) and other bacteria, yeasts and molds are key determinants for the production of fermented milk products with different tastes and flavors [1,2]. Fermented milks are beneficial to human health, conditioning the intestine environment, lowering the blood pressure, and reducing the risks of bladder cancer and colon cancer [3-8]. Nowadays, the increasing consumption of fermented milks offers a potential market for novel fermented milk products [9].Globally among the commercial fermented milk products, yogurt is the most popular product. Yogurt belongs to the thermophilic homolactic (acid) fermentation type of fermented milks since two thermophilic LAB species, Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus, are used together as the components of a yogurt starter [2]. An associative growth (protocooperation) has been observed during fermentation between these two bacterial species. Based on the literature, growths factors such as carbon dioxide (CO2) and formic acid produced by Streptococcus thermophilus, and particular amino acids especially valine provided by Lactobacillus delbrueckii ssp. bulgaricus proteolysis, are responsible for the observed synergistic effects [10-15]. Furthermore, it has been reported that formic acid is produced as a degradation product of lactose when milk bases are subjected to severe heat treatments [2].
Geotrichum candidum is an anamorphic and filamentous yeast like mold [16]. It can grow at temperatures ranging from 5 to 38°C, with an optimal growth at around 25°C. Growth occurs within a large pH interval from 3 to 11, with an optimal interval at 5.0-5.5. Besides the long lag phase, the generation time of G. candidum is 66 min in liquid culture at 30°C, being one of the shortest among eukaryotes, with final counts (tfu, thallus forming units) lower than 106 tfu/g [17,18]. G. candidum is usually involved in the ripening of various mold cheeses. Among various types of fermented milks “Viili”, a traditional fermented milk product mainly produced and consumed in Finland and in Sweden, is unique as its production is based on the use of a “Viili” starter containing both mesophilic LAB and G. candidum mold strains [2,19,20]. Unfortunately during the last 20 years the consumption of traditional “Viili” has declined to the benefit of a higher consumption of yogurt in Finland.
In the present study, we investigated whether there was a microbiological basis for the conception of a novel type of fermented milk, which would be a “hybrid” product between “Viili” and yogurt. In this kind of fermented milk, the mesophilic LAB of traditional “Viili” starter strains would be replaced by a thermophilic Lactobacillus delbrueckii strain or strains. In other words, the study consisted in examining whether the Streptococcus thermophilus species of the yogurt starter could be replaced by the G. candidum species to achieve successful milk fermentation.
Materials and Methods
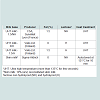
Skim milk powder, Yeast extract, M17 broth, MRS broth, sodium bicarbonate, sodium formate and disodium carbonate were purchased from Sigma-Aldrich (St. Louis, USA). M17 agar and yeast extract glucose chloramphenicol agar (YGC agar) were supplied by Merck (Darmstadt, Germany). MRS agar was from Lab M Ltd. (Lancashire, UK). The features of the milk bases are listed in Table 1.
Stock cultures of strains Lactobacillus delbrueckii ssp. lactis ATCC 15808, Streptococcus thermophilus T101 (Valio Ltd., Finland) and Geotrichum candidum VV (isolated from the “Viili” product manufactured by Valio Ltd., Finland) were preserved at -20°C.
Overnight cultures of strains ATCC 15808, T101 and VV were prepared in MRS, M17 and YGC broths, respectively. Strains ATCC 15808 and T101 were grown at 37°C, and strain VV at 30°C. The cell pellets were obtained by centrifugation of the cultures at 4000 rpm/5min followed by washing of the culture pellets twice with 0.85% NaCl solution. The cell pellets were subsequently resuspended into 2 mL 0.85% NaCl solution. Inoculation volumes of 100 μL for both ATCC 15808 and T101, and 150 μL for VV cell suspensions were transferred to 10 mL of the growth medium base. Both YGC and MRS broths were tested for the growth studies of G. candidum VV. Cultures were incubated at the considered temperature ranges. Bacterial growth was evaluated by (i) the enumeration of viable cells on agar plates, and (ii) the pH measurement of the cultures. The platings for the strains ATCC 15808 and T101 were performed on MRS-pH 5.4 agar incubated anaerobically, and on M17 agar incubated aerobically at 37°C, respectively. Anaerobic incubation conditions occurred in jars with AnaeroGenTM sachets (Oxoid Ltd., Hants, UK). The growth of G. candidum VV was determined on YGC agar under aerobic conditions at 30°C.
Results
Determination of an Optimal Growth Temperature for the Co-Culture of Lb. delbrueckii ssp. lactis ATCC 15808 and G.candidum VVTo investigate synergistic type of interactions, an overlap of the temperature ranges allowing the growth of both components of a co-culture is of a crucial importance. Accordingly, the growths of Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV were followed separately in MRS broth during 48 hours, in a temperature range from 30°C to 42°C. Two initial inoculation levels (high and low) were included in both cases. The results of these growth tests have been summarized in Figure 1. In all conditions, the incubation time of 24 hours was sufficient to reach the maximal level of viable cells in the culture. Especially with high initial inoculation levels, significant losses of G. candidum cell viability were observed at 37°C and 42°C (Figure 1A). The optimal growth temperature for G. candidum VV was 30°C, the lowest among the tested temperatures. Above 34°C, the growth was seriously if not completely prevented (Figure 1A and B). The thermophilic Lb. delbrueckii ssp. lactis ATCC 15808 could grow equally well at any of the tested incubation temperatures (Figure 1C and D). The best conditions for cell survival could be reached at 30°C and 32°C when a high initial inoculation level was tested (Figure 1C). Consequently, 30°C was selected as the optimal temperature to grow simultaneously Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV in a co-culture.
Figure 1: Determination of an optimal growth temperature for G. candidum VV (A and B) and Lb. delbrueckii ssp. lactis ATCC 15808 (C and D) grown in MRS broth. The enumeration of the cells was performed on MRS (pH 5.4) plates for strain ATCC 15808 and on YGC plates for strain VV at 37°C and 30°C, respectively, after 24 h and 48 h incubations. The horizontal line refers to the initial inoculation level applied (A and C: high level, B and D: low level).
Three milk bases -UHT milk-0/H (zero fat and hydrolyzed lactose) with or without autoclaving at 121°C for 10 minutes, and autoclaved (121°C for 10 minutes) reconstituted skim milk- were used for the investigations of possible synergistic interactions between Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV strains (Table 1). Low and high inoculation levels of VV compared to the levels of ATCC 15808 (t.f.u./ml:c.f.u./ml -ratios of 1:100 and 1:10, respectively) were tested; the growth of each culture at 30°C was followed by pH-measurements (Figure 2) and by specific platings for each component of the co-culture (Figure 3). A yoghurt starter like co-culture, consisting of Lb. delbrueckii ssp. lactis ATCC 15808 and Str. thermophilus T101 strains (for which the initial inoculation ratio of T101:ATCC 15808 was about 1:20), as well as the single strain cultures were used as references.
Figure 2: pH values of the co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV (A - C), and a yogurt starter like reference co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and S. thermophilus T101 (D - F) as incubated at 30°C for 48 hours. Three milk bases as culture media were tested: Autoclaved (121°C/10 min) reconstituted skim milk (A and D), autoclaved (121°C/10 min) fatless, lactose hydrolyzed (0/H) UHT milk (B and E), and fatless, lactose hydrolyzed (0/H) UHT milk (C and F). Two inoculation levels of VV were included: 1) Low level: the ratio of VV:ATCC 15808 was about 1:100 2) High level: the ratio of VV:ATCC 15808 was about 1:10. The pH values were determined from three replicates.
As shown in Figure 2, the acid production (drop of pH-value) in the co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV with the high initial inoculation ratio of VV:ATCC 15808 (1:10) was comparable to the yoghurt starter like reference coculture, Lb. delbrueckii ssp. lactis ATCC 15808 and S. thermophilus T101, at any time points, and in all three milk bases. This was also the case with the low initial inoculation ratio of VV:ATCC 15808 (1:100) for two autoclaved milk bases (Figure 2A and B). But, in the nonautoclaved UHT milk, the lower initial inoculum level of G. candidum VV retarded acid production compared to the higher inoculum of G. candidum VV (Figure C). Interestingly, just in this milk base, the presence of G. candidum VV, at either low or high inoculation levels, stimulated the growth of Lb. delbrueckii ssp. lactis ATCC 15808 as detected from the pH values of the cultures. Noteworthy, the trend of the drop of the pH values over time, reflecting the synergistic growth of Lb. delbrueckii ssp. lactis ATCC 15808 together with either G. candidum VV (high inoculum) (Figure 2C), or with Str. thermophilus T101 (Figure 2F) are fairly similar suggesting a comparable synergistic capability. Among the three considered milk bases, differences at c.f.u./ml or t.f.u./ml levels over time were most noticeable for either Lb. delbrueckii ssp. lactis ATCC 15808, or for G. candidum VV, when the growth studies were performed in nonautoclaved UHT milk (Figure 3). The growth of Lb. delbrueckii ssp. lactis ATCC 15808 was stimulated by the presence of G. candidum VV at either low or high inoculum; the growth of ATCC 15808 increased by about two log units in the co-cultures (conditions b and c in Figure 3A), whereas a rise of about one log unit was observed when ATCC 15808 was grown alone (condition a in Figure 3A). The stimulatory effect by G. candidum VV looked equivalent to the one promoted by Str. thermophilus T101 (condition d in Figure 3A). The same non-autoclaved UHT milk enabled the growth of G. candidum VV, at either low or high inoculum, by about one log unit in the presence of Lb. delbrueckii ssp. lactis strain ATCC 15808 (Figure 3B). No real differences in the growth of Str. thermophilus T101 were observed with the tested milk bases, in the presence or absence of Lb. delbrueckii ssp. lactis ATCC 15808 (Figure 3C).
Figure 3: Growth of the co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV (A and B), and a yogurt starter like reference co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and Str. thermophilcus T101 as incubated at 30°C for 24 hours. Milk bases and inoculation levels of VV were as described in Figure 2. Platings for counting Lb. delbrueckii, Str. thermophilus and G. candidum were done on MRS (pH 5.4), M17 and YGC agars as described in Materials and Methods.
As noticeable in Figures 2 and 3, the synergistic effects observed in the co-cultures of Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV strains, and of ATCC 15808 and Str. thermophilus T101 strains were most obvious when the milk base was not subjected to severe heat treatments like autoclaving at 121°C for 10 minutes. In other words, strong heat treatments of the milk base appeared to yield some compound(s), which promoted the growth of Lb. delbrueckii ssp. lactis ATCC 15808. The most obvious candidate is formic acid: it is known that formic acid is one of the major growth promoting metabolite delivered by Str. thermophilus, and it is required for the biosynthesis of purines [12,14,15,21]. Moreover, formic acid is also known to be one of the thermal degradation products of lactose [2]. Hence, we investigated whether formic acid would be a growth factor for Lb. delbrueckii ssp. lactis ATCC 15808. Based on the literature [15] and on our preliminary tests with 0.7 to 14.7 mM Na-formate (a dissociated form of formic acid, which exists at the pH of milk) supplements (data not shown), 7.35 mM Na-formate was chosen as the final formate supplement concentration in UHT milk (a nonautoclaved milk base) and in skim milk (autoclaved at 121°C for 10 min) for our growth factor analysis, performed both at 30°C and 42°C.
As revealed through pH measurements, growth promoting effectsby 7.35 mM formate on Lb. delbrueckii ssp. lactis ATCC 15808 were only observed in the case of the non-autoclaved UHT milk base, at both temperatures (Figure 4A and B). The addition of formate, to autoclaved skim milk, did not show any stimulatory effect on the growth of ATCC 15808 at both investigated temperatures (Figure 4C and D). Altogether these results suggest that the autoclaving heat treatment of skim milk had replaced the formate through suggested partial thermal lactose degradation. Consequently, the effects of the autoclave heat treatment (121°C for 10 min) of milk bases and a 7.35 mM formate supplement on the growth of Lb. delbrueckii ssp. lactis ATCC 15808 were compared in two UHT milk bases: UHT milk with non-hydrolyzed (NH) lactose, and UHT milk with hydrolyzed (H) lactose, having both similar fat content (1.5%). As shown in Figure 5, the autoclaving heat treatment caused similar growth promoting effects on Lb. delbrueckii ssp. lactis ATCC 15808, as did the 7.35 mM formate supplement for the UHT milks considered here. Altogether the growth promoting effect of the 7.35 mM formate supplement on Lb. delbrueckii ssp. lactis ATCC 15808 was independent on fat content, lactose hydrolysis or growth temperature.
Supplements of 5% CO2 in air, 10-100 mM NaHCO3 and 5-100 mM Na2CO3 in skim milk were tested as growth factors for Lb. delbrueckii ssp. lactis ATCC 15808. None of these additional compounds tested-carbon dioxide in gas phase or dissolved inorganic (bi) carbonate - did promote the growth of strain ATCC 15808 (data not shown).
Discussion
We investigated here whether there was a microbiological basis for the construction of a novel type of fermented milk, which would be a “hybrid” product between “Viili” and yogurt. A traditional “Viili” starter is based on the combination of mesophilic Lactococcus and Leuconostoc strains and Geotrichum candidum, a yeast-like white mold. LAB strains are responsible for lactic fermentation, citrate-based aroma formation and for characteristic high viscosity and ropiness of the “Viili” product. G. candidum mold creates a velvet-like creamy surface on “Viili”. Typically, in the manufacture of “Viili”, the fermentation takes place in the package and lasts about 18 to 20 h at 18-20°C [22]. On the other hand in the (set or stirred) yoghurt manufacture, the fermentation by a yogurt starter, which contains thermophilic Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus strains, typically lasts 2 to 4 h at 42-44°C, or longer times at lower temperatures [2,23]. Yogurt starter species, the growth of which is known to show mutual stimulation, are responsible for lactic fermentation, and productions of yogurt aroma(especially acetaldehyde) and possible exopolysaccharides (EPSs) [2].The replacement of the mesophilic LAB strains in a typical “Viili” starter by a single thermophilic Lb. delbrueckii strain, and the subsequent combination of this single strain with G. candidum was possible as the strains ATCC 15808 and VV shared a temperature range of 30-34°C (Figure 1), which allowed the simultaneous growth of both strains; we selected 30°C as the optimal temperature. This choice is consistent with the literature data, which reports that 100% of the G. candidum isolates were able to grow at 30°C, but only 1% of the isolates grew at 37°C [24].
From the results in Figures 2 and 3, the co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and G. candidum VV showed similar synergistic properties as the yogurt starter like reference co-culture of Lb. delbrueckii ssp. lactis ATCC 15808 and Str. thermophilus T101 when growing at 30°C in various milk bases. Formic acid (present as formate at the pH of the milk) and CO2 produced by Str. thermophilus have been described to be the major compounds, which promote the growth of the second yogurt starter species, Lb. delbrueckii ssp. bulgaricus [2,25]. We demonstrated that formate, supplemented at 7.35 mM, could substitute the growth promoting effect of Str. thermophilus T101 or of G. candidum VV on Lb. delbrueckii ssp. lactis ATCC 15808 in UHT milk, but not anymore in skim milk, which had been autoclaved at 121°C for 10 minutes (Figure 4). As shown in Figure 5, a severe heat treatment like the autoclaving at 121°C for 10 minutes of the milk base was able to replace the formate supplement, obviously due to formic acid formation, through partial thermal lactose degradation [2]. Based on our observations, we suggest that G. candidum is able to replace successfully Str. thermophilus for the metabolic formic acid production required for the growth of Lb. delbrueckii. We could not observe any stimulatory effects on the growth of Lb. delbrueckii ssp. lactis ATCC 15808 by (bi) carbonate or CO2 supplements (data not shown), even though the respirative metabolism by G. candidum, required for its growth, generates CO2 at the cost of dissolved oxygen (O2). G. candidum cannot metabolize lactose; but lactose fermentation by Lb. delbrueckii will produce a suitable carbon source, lactic acid, for the growth of G. candidum in the co-culture in a milk base. In addition to formic acid, G. candidum may synthesize other compounds, like unsaturated fatty acids, which are essential for the growth of Lb. delbrueckii [26]. Furthermore G. candidum may create an anaerobic environment for Lb. delbrueckii by consuming dissolved oxygen in the milk base, and by that way improving the growing conditions of the bacterial partner.
In this study, we have shown that a “Termoviili” starter like coculture consisting of Lb. delbrueckii ssp. lactis and G. candidum strains is exhibiting similar synergistic properties as a yogurt starter like coculture when growing in a milk base, where no or very low thermal lactose degradation occurred. The manufacture of “Termoviili” would take about the same time as the manufacture of the traditional “Viili”, but the incubation temperature should be about 10-12°C higher, around 30°C. Contrarily to the standard yogurt manufacture, the milk for “Termoviili” should not be homogenized, and the fermentation should happen in the package in order to produce the velvet like cream layer covered with G. candidum in the final product like in the case of “Viili”. Of crucial importance for the success of a novel type of fermented milk are its sensory and nutritional properties. Our very preliminary data on sensory properties of “Termoviili” indicated a flavor with slightly sharp, light alcohol, yeast/musty and organic acid flavor features, judged different from a typical yogurt flavor, but acceptable by all five panel members (data not shown).
Figure 4: Effects of the 7.35 mM sodium formate (HCOONa) supplement in UHT milk (non-autoclaved) (A and B) and skim milk (autoclaved at 121°C for 10 min) (C and D) on the growth of Lb. delbrueckii ssp. lactis ATCC 15808 incubated at 42°C (A and C) and 30°C (B and D) as based on pH-values of the cultures. UHT milk-1.5/NH: lactose non-hydrolyzed UHT milk containing 1.5% fat; skim milk: reconstituted (10% w/v) skim milk (autoclaved at 121°C for 10 minutes).
Figure 5: Effects of autoclave heat treatment (121°C for 10 minutes) of lactose hydrolyzed and lactose non-hydrolyzed UHT milks containing 1.5% fat (UHT milk-1.5/H and UHT milk-1.5/NH, respectively) on the growth of Lb. delbrueckii ssp. lactis ATCC 15808 incubated at 30°C as based on pH-values of the cultures.
At first, the manufacture of “Termoviili” would be as simple as “Viili”; but because there is no need to homogenize the milk fat, and because there is no need to control the ratio of the two thermophilic LAB starter species during milk fermentation due to the elimination of Str. thermophilus, one could consider milk less processed, and the manufacture of “Termoviili” hence looks even simpler than the manufacture of yogurt, even for a set type yogurt. Further studies with additional starter strains of both Lb. delbrueckii and G. candidum are needed to develop optimal starters, and finally a novel fermented milk product, “Termoviili”.
References
- Tamime AY (2002) Fermented milks: a historical food with modern applications -- a review. Eur J Clin Nutr 56: S2-S15.
- Walstra P, Wouters JTM, Geurts TJ (2006) Dairy Science and Technology. (2nd edtn), Taylor & Francis CRC Press.
- Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H et al. (2001) Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int 68: 273-280.
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJ (2003) Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol 17: 655-659.
- Seppo L, Jauhiainen T, Poussa T, Korpela R (2003) A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 77: 326-330.
- Larsson SC, Andersson S, Johansson J, Wolk A (2008) Cultured milk, yoghurt, and daily intake in relation to bladder cancer risk in a prospective study of Sweden women and men. Am J Clin Nutr 88: 1083-1087.
- Matsumoto K, Takada T, Shimizu K, Moriyama K, Kawakami K et al.(2010) Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environmental of healthy individuals with soft stools. J Biosci Bioeng 110: 547-552.
- Pihlanto A, Virtanen T, Korhonen H (2010) Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int Dairy J 20: 3-10.
- IDF (2011) The world dairy situation 2011. IDF Bulletin Volume 451: 225.
- Driessen FM, Kingma F, Stadhouders, J (1982) Evidence that Lactobacillus bulgaricus in yoghurt is stimulated by carbon dioxide produced by Streptococcus thermophilus. Neth Milk Dairy J 36: 135-144.
- Ginovart M, Lopez D, Valls J, Silbet M (2002) Stimulation modeling of bacterial growth in yoghurt. Int J Food Microbiol 73: 415-425.
- Courtin P, Rul F (2003) Interactions between microorganisms in a simple ecosystem: yoghurt bacteria as a study model. Lait 84: 125-134.
- Crittenden RG, Martinez NR, Playne MJ (2003) Synthesis and utilization of folate by yoghurt starter cultures and probiotic bacteria. Int J Food Microbiol 80: 217-222.
- Sieuwerts S, Bok FAM, Hugenholtz J, Hylckama Vileg JET (2008) Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl Environ Microbiol 74: 4997-5007.
- Siewerts S, Molenaar D, Hijum SAFT, Beerthuyzen M, Stevens MJA, et al. (2010) Mixed-culture transcriptome analysis reveals the molecular basis of mixed-culture in Streptococcus thermophilus and Lactobacillus bulgaricus. Appl Environ Microbiol 76: 7775-7784.
- Missous G, Thammavongs B, Launay G, Gueguen M Dieuleveux V, et al.(2010) Relationship between growth behaviour, micro- and macroscopic morphologies and freeze sensitivity of the ripening starter Geotrichum candidum is strain specific and mostly related to the morphotypes: the arthrospores/hyphae parameter. J Dairy Res 77: 425-431.
- Boutrou R, Gueguen M (2005) Interests in Geotrichum candidum for cheese technology. Int J Food Microbiol 102: 1-20.
- Hudevoca A, Valik L, Liptakova D (2009) Quantification of Geotrichum candidum growth in co-culture with lactic acid bacteria. Czech J Food Sci 27: 18-27.
- Law BA (1997) Microbiology and Biochemistry of Cheese and FermentedMilk. (2nd edtn), Blackie Academic & Professional, London.
- Marcellino N, Beuvier E, Grappin R, Gueguen M, Benson DR (2001) Diversity of Geotrichum candidum strains isolated from traditional cheesemaking fabrications in France. Appl Environ Microbiol 67: 4752-4759.
- Derzelle S, Bolotin A, Mistou M, Rul F (2005) Proteome analysis of Streptococcus thermophilus grown in milk reveals pyruvated formate-lyase as the major upregulated protein. Appl Environ Microbiol 71: 8597-8605.
- Roginski H (2002) Fermented milk: Northern Europe. Encyclopedia of Dairy Sciences 2: 1034-1041, Academic Press.
- Robinson H (2002) Fermented milks: Yoghurt types and manufacture. Encyclopedia of Dairy Sciences 2: 1055-1058, Academic Press.
- Eliskases-Lechner F (2002) Geotrichum candidum. Encyclopedia of Dairy Sciences, 2: 1229-1234, Academic Press.
- Robinson H (2002) Fermented milks: Yoghurt, role of starter cultures. Encyclopedia of Dairy Sciences Vol 2: 1059-1063, Academic Press.
- Partanen L, Marttinen N, Alatossava T (2001) Fats and fatty acids as growth factors for Lactobacillus delbrueckii. Syst Appl Microbiol 24: 500-506.