Journal of Clinical Trials & Patenting
Download PDF
Research Article
Clinical Study to Evaluate Safety and Efficacy of a Topical Hair Minimizing Lotion in Healthy Human Volunteers
Kondhalkar Mrinmayee1*, Kishori Apte1, PradeepParab1, Anagha Dudhbhate2 and Rinti Banerjee3
- 1APT Research Foundation, Pune, India
- 2Deenanath Mangeshkar Hospital, Pune, India
- 3Indian Institute of Technology, Mumbai, India
*Address for Correspondence:Kondhalkar Mrinmayee Bhushan, APT Research Foundation, Pune, India, Tel: +91 9371 290 971; E-mail: mrinmayee.bhushan@gmail.com
Citation: Kondhalkar M, Dudhbhate A, Apte K, Banerjee R, Parab P. Clinical Study to Evaluate Safety and Efficacy of a Topical Hair Minimizing Lotion in Healthy Human Volunteers. J Clin Trials Pat 2017;2(1): 4.
Copyright © 2017 Kondhalkar M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Trials& Patenting | ISSN: 2573-3834 | Volume: 2, Issue: 1
Submission: 1 April, 2017| Accepted: 13 May, 2017 | Published: 18 May, 2017
Abstract
Background: Management of unwanted hair growth arising due to hirsutism or hypertrichosis is a huge challenge. Prevalence rate of hirsutism due to endocrine disorders is very high with very few effective treatment options.
Aims: Primary objective of this study was to evaluate safety of testmaterial with repeated applications according to Draize and Use Test. Secondary objective of this study according to the Use Test was to evaluate hair growth inhibition potential of test material.
Methods: Thirty four healthy volunteers were enrolled in an unicentric double blind, self-controlled clinical study to evaluate safety and efficacy of Romantaque according to Draize test and Use Test according to IS4011:1997/2009 guidelines for a period of one year. Efficacy was evaluated by Use Test according to IS4011:1997/2009 guidelines by phototrichogram. The data is analyzed using paired T test (two tailed).
Results: All thirty four volunteers completed Draize Test and test material was found to be safe for use. Thirty volunteers were enrolled and completed the Use Test. None of the volunteers showed any adverse events during safety evaluations for Use Test. Efficacy evaluations for Use Test showed that the test group (“Romantaque Lotion”) showed statistically significant hair growth inhibition. Also this test group consistently showed a dose response of reduction in number of hair at every time point as compared to pre-treatment levels. The differences between diameter of hair on Control and Test areas with respect to before and after treatment for all subjects was found to be statistically very significantly (p<0.0001***).
Keywords
Hirsutism; Unwanted hair; Hypertrichosis; Hair inhibition; PCOS
Introduction
Hirsutism affects five to ten percent of female population [1]. There is a strong correlation between hirsutism and other clinical conditions such as the polycystic ovary syndrome (PCOS), or syndromes of severe insulin resistance [2,3]. Over 40% of adult women suffering from unwanted facial hair growth, it’s the psychological and psychosocial impact should not be underestimated [1].
Considering all available treatment options for the management of hirsutism, there is urgent need for new therapeutic strategies such as new biological response modifiers offering more effective means to reduce and manage unwanted body and facial hair [1]. Though different medications with inconclusive efficacies have been used for long, no definitive treatment has been introduced yet.
This prompted us to assess and validate a new therapeutic FDA (MAH- India) approved topical Romantaque Lotion
Description of test material
Name: Romantaque - Post Hair Removal Hair minimizing Lotion Composition: Aqua (Water), Cetostearyl Alchohol, Cetyl Alchohol, Liquid Paraffin, Methyl Paraben, Propyl Paraben, Stearic Acid, Sodium Alginate, Castor extract, Triethanolamine
Material Category: Topical Cosmetic
Manufacturing license no- PD/C-27A, Batch no- RT1201
Manufacturing Date: July 2012 Expiry date: June 2014
Romantaque is indicated for use 24 hours after hair the hair is removed from the follicle for ten days, twice a day. This study was designed to generate qualitative as well as quantitative data to prove the safety of “Romantaque Lotion” and further efficacy of this cosmetic product in healthy females.
Materials and Methods
The study was conducted in accordance with the IS4011:1997/2009 guidelines. According to IS 4011:1997/2009, the minimum sample size of the study population for conducting safety in healthy human volunteers is twenty four. Hence, thirty four healthy volunteers with age group of 18 to 60 were recruited for the study, with study duration of about five months per subject.
This study was a randomized, parallel group, double blind, placebo controlled trial for assessing the safety and efficacy of cosmetic product Romantaque Lotion in healthy human volunteers according to IS 4011:1997/2009 Guidelines for three hair growth cycles. This clinical study included two protocols to evaluate safety and efficacy of the test material, Draize test- (RMTK 01) and Use Test- (RMTK 02). The study was carried out between 15 March 2013 and 13 February 2014 at National Toxicology Centre, Pune. The study protocol was approved by an Independent Ethics Committee, Informed written consent were obtained from all the volunteers before enrollment for study. This study has been registered with Clinical Trials Registry of India (CTRI) - CTRI/2015/02/005534.
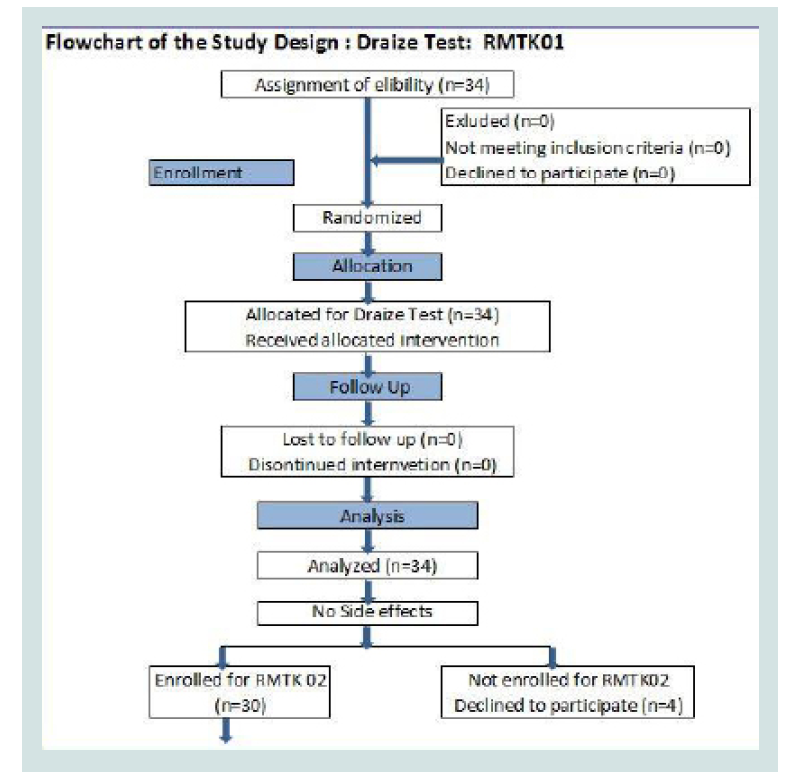
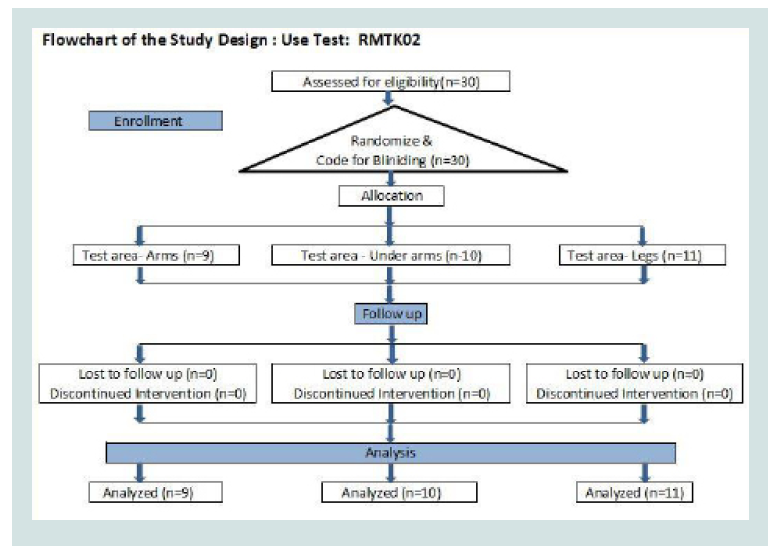
Volunteers: Volunteers were enrolled from willing population of adult females from Pune, Maharashtra, India. Inclusion criteria included clinically healthy adult females between the age group of 18 to 60, without any known illness, infection or allergy. Volunteers with known skin disease, lactating mothers, pregnant women and those planning to get pregnant within six months, diabetic, malignant, hypertensive, or those on any other treatment were excluded from the study. A flowchart of study design has been shown as per the CONSORT guidelines (Tables 1 and 2) [4].
Test material: Romantaque Lotion is a commercial cosmetic product recommended for post hair removal hair minimizing lotion. The active agent of this product is castor extract. Castor extract constitutes cytotoxic type II ribosome inactivating protein ricin. Ricin was earlier shown to inhibit the growth of hair follicles without affecting any other skin structures [a href="#ref5">5,6]. Though the exact molecular mechanisms involved are yet to be elucidated, considering the ribosome inactivating and cytotoxic nature of ricin it may be interfering in cellular process of hair shaft synthesis. This interference is observed in physiological evidence in hair follicle dystrophy [5,6].
Draize test: The skin was to be exposed to the Test Material. For this, ventral skin of forearm of each subject enrolled was selected. There were three test areas for each individual subject. Right forearm of each subject had one test area; it was referred as ‘Control/C’, while left forearm had two test areas, and was referred as ‘Patch with drug /D’ & ‘Only Patch/ P’. For accurate occlusive application, suitably made aluminium cups of around one cm diameter were fixed to the forearm by a surgical adhesive tape. The skin was exposed to Test Material 24 hrs for first day. The rating of skin reactions as per Draize Test were recorded after completion of 48 hrs of initial application During the exposure free intervals the test area was not protected or treated in any other way.
Use test: Randomization
All subjects were selected among the willing population. As this study was self-control study, first 34 Subjects were enrolled and put in three groups designated as Forearm, Axilla, and Legs according to test area. First ten volunteers from the willing population were enrolled in each group according to their preference of test group. Subjects were to receive control and “Romantaque Lotion” on either left or right test area.
After enrolment hair was removed by waxing from the test areas of subject. All test areas were observed for hair growth before application of test material.
Subjects were educated and trained how to apply the lotion on respective test areas once daily for 15 days starting 24 hrs after waxing and to fill up the patient’s diary to note down the Adverse Events (AE) if any.
All adverse/side effects, adverse events and effect of study Material were documented on follow up form. All test areas were observed for hair growth and photographs (Trichoscan) by digital microscope were taken.
Subjects were instructed to remove hair on the test areas by waxing and repeat the regimen of repeated application of test material on waxed test areas according to the designated codes till next expected hair growth. One independent investigator allocated volunteer codes and monitored the allocation of blinded containers to volunteers. This investigator also analyzed the data generated (phototrichograms) by principal investigator. The principal investigator was blinded with respect to allocated test and control areas to volunteers. The volunteers too were blinded with respect to the identity of coded formulation containers.
Results
Draize test: All thirty four subjects enrolled for this study, completed the study. None of the subjects showed either of the Adverse Events (AE)/Serious Adverse Events (SAE) symptoms or other symptoms of Draize test at all points. These results confirm the safety of “Romantaque Lotion” in healthy volunteers.
Use test: Four volunteers discontinued from the study voluntarily as they expressed their inability to continue to follow the study regimen. Thirty volunteers completed this study.
As the study was designed as Use Test extended for three hair growth cycles, this study provided data for long term safety and efficacy of “Romantaque Lotion”.
Primary objective of Use Test for safety evaluations showed that none of the volunteers showed any AE/SAE symptoms related to product use. One subject showed minor rash due to waxing.
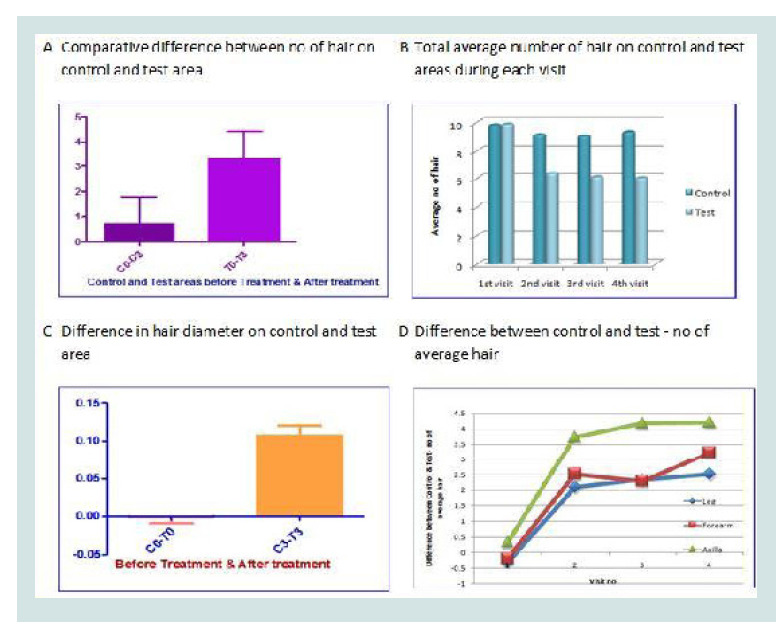
As a secondary objective of Use Test to assess evaluations, when the difference between number of hair on Control and Test areas were compared at pre-treatment and post-treatment level using paired T test, it showed statistically significant reduction (n=30, t=6.494, p<0.0001) in number of hair in test areas (Figure 1A). The test group in which “Romantaque Lotion” was applied showed hair growth inhibition and reduced number of hair at every time point as compared to pre-treatment levels of assessment (Figure 1B).
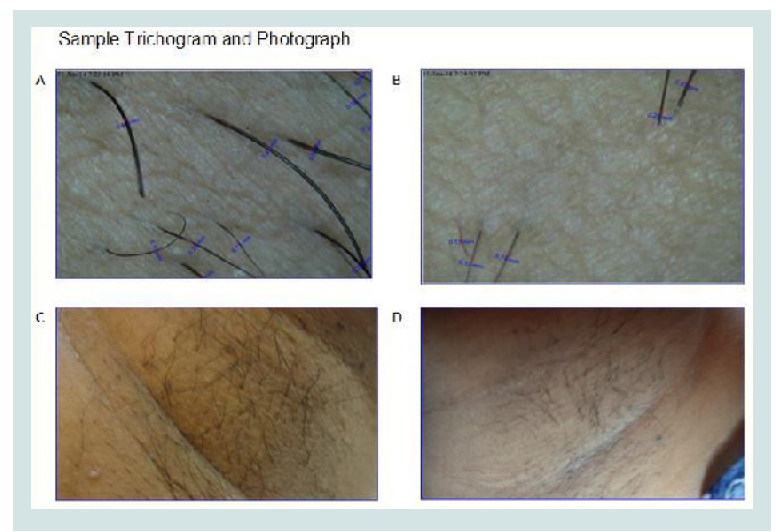
Figure 1: (A) Difference in number of hair with respect to control areas (C0) and test areas (T0) at pre-treatment level; and at the end of the treatment (C3) and (T3) respectively. (B) At every visit the number difference in the number of hair at each time point goes on increasing when compared to pretreatment level (first visit) (n=30). (C) Difference between in diameter of hair in control areas (C0) and in test areas (T0) at pre-treatment level; and at the end of the treatment (C3) and (T3) respectively. (D) Sample control area (A) shows more number of hair with more diameter as compared to the sample test area (B) at the end of the treatment (original).
Similarly, when the difference between diameter of hair on Control and Test areas were compared at before and after treatment (C0-T0 and C0-T0) for all subjects showed significant (n= 26) (t=8.116, p<0.0001***) increase in the difference showing very effective reduction in diameter of hair in test areas (Figure 1C). The data is analyzed using paired T test (two tailed). The response of the hair follicles to the treatment with Romantaque varies with location of the hair follicles. When the difference between number of hair on Control and Test areas were compared at each time points for all three test groups according to test areas (Forearms, Axilla and Legs) showed significant difference in number of hair in test areas, Axilla (t=3.732, p<0.0047**), Forearm (t=3.648, p<0.0065**) and Legs (t=4.660, p<0.0009***). The data is analyzed using paired T test (two tailed) (Figure 1D) [7].
“Romantaque Lotion”, applied once daily for fifteen days after waxing for three hair growth cycles was well tolerated and significantly reduced the number and thickness of unwanted hair on forearms, axilla and legs, as measured by a dermatologist’s assessment and trichogram (three locations in a test area) by using the in-built software of the Digital microscope (Figure 2).
Discussion
Based on the findings of safety and efficacy, it can be concluded that the test material “Romantaque Lotion” is safe for use and very effective.
The time response profile observed with “Romantaque Lotion” suggests that a more pronounced efficacy may be achieved with consistent and longer treatment. The treatment profiles observed for different body areas showed the potential of this product to significantly minimize unwanted hair growth qualitatively and quantitatively.
Though ricin was known to be dermally inactive as absorption amount was insignificant [8,9], recently ricin extracted from castor (Ricinus communis) has been shown to inhibit the growth of hairfollicles by inducing hair follicle dystrophy [5,6]. The same mechanism may be proposed to be working in case of the test formulation as the active agent in the formulation is castor extract. The response of the hair follicles to the treatment varies with location of the hair follicles, as the nature and location of stem cells may differ betweenfollicle types [10]. This current study observation is concurrent with observations made earlier and showed that response of hair follicles to the treatment varies with the location of the follicles.
Increasing unforgiving nature of modern society towards hirsutism and hypertrichosis forces affected women to seek desperately all possible hair removal methods, whether temporary, long term or permanent in the form of pumice stone, depilatories, shaving, waxing, sugaring, threading, electrolysis, laser or antiandrogens [1,11], Not just the cosmetic need of the patient, but it is imperative to assess the impact on her quality of life in the form of social phobia, disfigurement anxiety, and depression [12]. Many female patients seek medical help for hirsutism only when it is of considerable severity [13]. The psychological need of the patients for privacy of treatment, and cost factor play a major deciding factor.
Thus, the clinical findings of this study endorse that Romantaque Lotion may provide simple, safe and effective treatment option of reducing unwanted body hair in conjunction with other methods of hair removal. It is imperative to remember that for management of unwanted hair growth regularity, consistency and persistence in treatment in very crucial for the success of the treatment.
Limitations
As hair growth patterns and responses of hair follicles to treatment varies from person to person, and according to location of the hair follicles, more extensive quantitative clinical studies are necessary to confirm the current findings. The study was designed to assess the efficacy of “Romantaque Lotion” as self-controlled study to minimize the error due to person to person variations in hair growth patterns. However, getting volunteers for assessment of effect on facial hair became a challenge as volunteers were reluctant for a self-controlled study design. Self-use by the volunteers may be considered as a bias.
References
- Azziz R, Carmina E, Sawaya ME (2000) Idiopathic hirsutism. Endocr Rev 21: 347-362.
- Sonthalia S, Bharti J (2014) Polycystic ovarian disease: still an enigma! Indian J Dermatol Venereol Leprol 80: 153-154.
- Madnani N, Khan K, Chauhan P, Parmar (2014) Polycystic ovarian syndrome: a review. Indian J Dermatol Venereol Leprol 80: 154-155.
- Schulz KF, Altman DG, Moher D, CONSORT Group (2010) CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 340: c332.
- Kondhalkar MB, Parab PB (2013) 7th World congress for hair research abstracts. J Invest Dermatol 133: 1391-1439.
- Kondhalkar MB, Apte K, Watve M, Paygude S, Parab P (2017) Ugly duckling or a swan: exploring therapeutic potential of ricin for inhibiting unwanted hair growth. Matters 3: 1410.
- Higgins PB, Fields DA, Hunter GR, Gower BA (2001) Effect of scalp and facial hair on air displacement plethysmography estimates of percentage of body fat. Obes Res 9: 326-330.
- Franz DR, Jaax NK (1997) Ricin Toxin. In: Zajtchuk R, Bellamy RF, (eds). Textbook of military medicine: medical aspects of chemical and biological warfare. TMM Publications, Washington, DC, USA, pp. 605-634.
- Schep LJ, Temple WA, Butt GA, Beasley MD (2009) Ricin as a weapon of mass terror--separating fact from fiction. Environ Int 35: 1267-1271.
- Stenn KS, Paus R (2001) Controls of hair follicle cycling. Physiol Rev 81: 449-494.
- Khanna N (1993) Cosmetic treatment of hirsutism. Indian J Dermatol Venereol Leprol 59: 109.
- Blume-Peytavi U, Gieler U, Hoffmann R, Lavery S, Shapiro J (2007) Unwanted facial hair: affects, effects and solutions. Dermatology 215: 139-146.
- Mathur SK, Ammini AC (1996) Body hair distribution of women attending endocrine OPD. Indian J Dermatol Venereol Leprol 62: 268.