Journal of Clinical and Investigative Dermatology
Download PDF
Verilich serum is a proprietary topical formulation containing [peptides, biotin, plant extracts, amino acids, vitamins]. While precise mechanisms were not investigated in this study, the formulation theoretically targets multiple pathways:
• Growth factor modulation supporting anagen phase maintenance
• Scalp microcirculation enhancement promoting follicular nutrition
• Anti-inflammatory effects beneficial in telogen effluvium
• Antioxidant properties protecting follicles from oxidative stress
Nutritional Supplementation: All patients received iron supplements (individualized dosing), vitamin D3 60,000 IU weekly for 3 months, and methylcobalamin 500mcg daily for 3 months. Patients with severe anemia (hemoglobin <10 g/dL, ferritin <10 ng/ mL) received IV iron (Orafer-FCM 500mL) bi-weekly for 2 cycles.
Condition-Specific Therapy:Male pattern hair loss patients received finasteride 1mg or oral minoxidil 2.5mg daily. Female pattern hair loss patients received topical minoxidil 2-5% or hormonal therapy as appropriate.
Secondary Endpoints:Safety/tolerability assessment, adverse event monitoring, patient satisfaction, photographic documentation.
Follow-up:Week 4 (initial response), Month 2 (primary endpoint), Month 3 (final assessment), Month 6 (pattern hair loss long-term follow-up).
Safety Monitoring
Systematic monitoring for local reactions (erythema, scaling, pruritus), systemic side effects (headache, dizziness, GI symptoms), contact sensitization, and drug interactions. All adverse events documented and graded for severity and relationship to treatment.
Statistical Analysis
Descriptive statistics summarized demographics and clinical characteristics. Treatment duration categorized as fast response (≤2 months) versus standard response (≥3 months). Comparison between chronic telogen effluvium and pattern hair loss groups using chisquare test (categorical variables) and independent t-test (continuous variables). P<0.05 considered statistically significant.
Quality Control
All assessments performed by the same dermatologist (Dr. Vimala Manne). Standardized photography protocol maintained (same camera, lighting, distance, angle). Regular dermoscopy equipment calibration. Patient compliance monitored through diary cards and product accountability.
Key Findings:
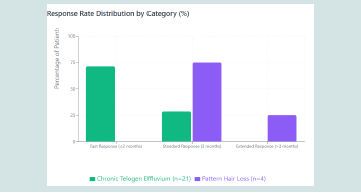
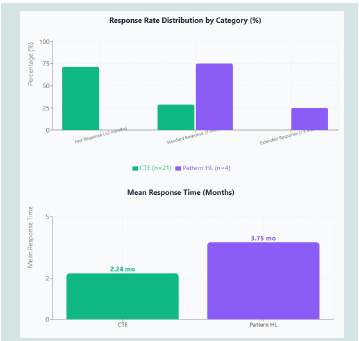
• 71.4% of chronic telogen effluvium patients (15/21) achieved clinical improvement within 2 months, defined as >50% reduction in daily hair shedding and negative hair pull test (<3 hairs extracted from three scalp areas)
• All pattern hair loss patients responded within 3-6 months with reduced hair loss and improved hair density
• Overall fast response rate: 60% (15/25)
Progressive clinical improvement in chronic telogen effluvium patients followed a consistent temporal pattern. Initial response emerged at week 3-4 (14.3%, 3/21 with negative hair pull test). By month 2, 71.4% (15/21) achieved the primary endpoint with significant shedding reduction and negative hair pull test. All 21 patients (100%) achieved clinical improvement by month 3.
Subgroup analysis of chronic telogen effluvium patients revealed consistent response patterns across demographics: female (73.3%, 11/15) versus male (66.7%, 4/6) fast responders (p=0.89); no significant differences by age groups (p=0.98). Treatment response was independent of gender, age, or symptom duration.
Two chronic telogen effluvium patients with severe anemia (hemoglobin <10 g/dL, ferritin <10 ng/mL) received additional IV iron therapy. Both achieved clinical improvement (one at 2 months, one at 3 months), demonstrating protocol efficacy even in complex cases requiring intensive nutritional rehabilitation.
The excellent compatibility with concurrent therapies is particularly relevant for clinical practice. The ability to incorporate Verilich serum alongside iron supplements, vitamins, finasteride, minoxidil, and hormonal therapy without drug interactions represents practical clinical advantage, supporting integration into diverse treatment strategies.
For pattern hair loss, while treatment duration remains consistent with conventional expectations, the excellent safety profile supports consideration of Verilich serum as a component of comprehensive strategies. This is particularly important given chronic therapy requirements where tolerability becomes critical for sustained compliance.
The small pattern hair loss sample size (n=4) limits generalizability for these conditions. Future studies should include larger pattern hair loss cohorts. Single-center design may limit generalizability, though demographic diversity of the chronic telogen effluvium cohort suggests broader applicability.
Long-term follow-up beyond 6 months would provide insights into response durability and optimal treatment duration. Randomized controlled trials with larger populations and separate treatment arms would strengthen evidence. Mechanistic studies investigating pathways through which Verilich serum may contribute to outcomes could inform future therapeutic optimization.
Research Article
Breaking the Hair-Loss Barrier: Pattern-Wise Efficacy of Verilich Serum
Manne V*
Department of Skin, Hair and Laser Center, Hyderabad, Telangana, India
*Address for Correspondence:Dr. Vimala Manne, Department of Skin, Hair and Laser Center,
Hyderabad, Telangana, India. E-mail Id: manne.vimala@gmail.com
Submission:12 December, 2025
Accepted:08 January, 2026
Published:12 January, 2026
Copyright: © 2026 Manne V. This is an open access article
distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in
any medium, provided the original work is properly cited.
Keywords:Chronic Telogen Effluvium; Male Pattern Hair Loss; Female
Pattern Hair Loss; Androgenetic Alopecia; Verilich Serum; Topical
Treatment, Hair Loss Management; Clinical Efficacy
Abstract
Background: Chronic telogen effluvium and pattern hair loss
requires prolonged treatment duration, typically 3-6 months with
conventional therapy. This study evaluated the efficacy and safety of
Verilich serum in enhancing clinical outcomes across multiple hair loss
conditions.
Methods: A prospective observational study included 25 patients: 21 with chronic telogen effluvium (15 females, 6 males; mean age 27.8 years), 2 with male pattern hair loss, and 2 with female pattern hair loss. All patients received Verilich serum 1mL daily application combined with nutritional supplementation (iron, vitamin D3 60,000 IU weekly, methylcobalamin 500mcg daily). Pattern hair loss patients additionally received hormonal therapy or minoxidil/finasteride as indicated. Primary endpoint was time to clinical improvement defined as >50% reduction in hair shedding and negative hair pull test (<3 hairs extracted).
Results: In chronic telogen effluvium patients, 71.4% (15/21) achieved clinical improvement within 2 months, characterized by significant reduction in daily hair shedding and conversion to negative hair pull test. All pattern hair loss patients demonstrated clinical response within 3-6 months with reduced hair loss and improved density. Overall, 60% (15/25) showed accelerated response (≤2 months). Zero adverse events were reported across all groups with 100% treatment completion.
Conclusion: Verilich serum demonstrated significant efficacy in accelerating clinical improvement in chronic telogen effluvium with favorable safety profile. These findings indicate that Verilich serum may play a beneficial role within comprehensive management strategies for diverse hair loss conditions.
Methods: A prospective observational study included 25 patients: 21 with chronic telogen effluvium (15 females, 6 males; mean age 27.8 years), 2 with male pattern hair loss, and 2 with female pattern hair loss. All patients received Verilich serum 1mL daily application combined with nutritional supplementation (iron, vitamin D3 60,000 IU weekly, methylcobalamin 500mcg daily). Pattern hair loss patients additionally received hormonal therapy or minoxidil/finasteride as indicated. Primary endpoint was time to clinical improvement defined as >50% reduction in hair shedding and negative hair pull test (<3 hairs extracted).
Results: In chronic telogen effluvium patients, 71.4% (15/21) achieved clinical improvement within 2 months, characterized by significant reduction in daily hair shedding and conversion to negative hair pull test. All pattern hair loss patients demonstrated clinical response within 3-6 months with reduced hair loss and improved density. Overall, 60% (15/25) showed accelerated response (≤2 months). Zero adverse events were reported across all groups with 100% treatment completion.
Conclusion: Verilich serum demonstrated significant efficacy in accelerating clinical improvement in chronic telogen effluvium with favorable safety profile. These findings indicate that Verilich serum may play a beneficial role within comprehensive management strategies for diverse hair loss conditions.
Introduction
Hair loss disorders, including chronic telogen effluvium (CTE),
male pattern hair loss (MPHL), and female pattern hair loss (FPHL),
represent common and psychologically distressing dermatological
conditions affecting millions worldwide [1,2]. These conditions share
the challenge of requiring prolonged treatment duration with slow
response to conventional therapies, significantly impacting patient
quality of life and compliance [3].
Chronic telogen effluvium, characterized by persistent hair shedding exceeding six months, predominantly affects women with an estimated prevalence of 2-5% [4]. The pathophysiology involves nutritional deficiencies (particularly iron and vitamin D), hormonal imbalances, psychological stress, and systemic conditions [5,6]. Pattern hair loss affects approximately 50% of men and 40% of women by age 50, involving genetic predisposition and androgen sensitivity [7,8].
Current therapeutic approaches vary by condition but share common limitations. Chronic telogen effluvium treatment focuses on nutritional supplementation with iron, vitamin D, and vitamin B complex [9]. Pattern hair loss management involves topical minoxidil, oral finasteride in males, and anti-androgenic therapy in females [10,11]. However, conventional protocols typically require 3-6 months to demonstrate clinical improvement, with many patients experiencing continued shedding during initial treatment, leading to poor compliance and psychological distress [12].
Chronic telogen effluvium, characterized by persistent hair shedding exceeding six months, predominantly affects women with an estimated prevalence of 2-5% [4]. The pathophysiology involves nutritional deficiencies (particularly iron and vitamin D), hormonal imbalances, psychological stress, and systemic conditions [5,6]. Pattern hair loss affects approximately 50% of men and 40% of women by age 50, involving genetic predisposition and androgen sensitivity [7,8].
Current therapeutic approaches vary by condition but share common limitations. Chronic telogen effluvium treatment focuses on nutritional supplementation with iron, vitamin D, and vitamin B complex [9]. Pattern hair loss management involves topical minoxidil, oral finasteride in males, and anti-androgenic therapy in females [10,11]. However, conventional protocols typically require 3-6 months to demonstrate clinical improvement, with many patients experiencing continued shedding during initial treatment, leading to poor compliance and psychological distress [12].
Recent advances in hair follicle biology have paved the way for
novel topical formulations that may enhance treatment outcomes
across multiple hair loss conditions [13]. Verilich serum represents
one such advancement, formulated with bioactive ingredients
designed to target multiple pathways involved in hair loss while
providing synergistic benefits when combined with conventional
therapies.
This study aimed to evaluate the clinical efficacy and safety of Verilich serum in enhancing treatment outcomes for patients with chronic telogen effluvium, male pattern hair loss, and female pattern hair loss. Specifically, we assessed whether incorporating Verilich serum into comprehensive treatment protocols could reduce time to clinical improvement from conventional 3-month timeline to 2 months, while maintaining excellent tolerability across different hair loss conditions [14].
This study aimed to evaluate the clinical efficacy and safety of Verilich serum in enhancing treatment outcomes for patients with chronic telogen effluvium, male pattern hair loss, and female pattern hair loss. Specifically, we assessed whether incorporating Verilich serum into comprehensive treatment protocols could reduce time to clinical improvement from conventional 3-month timeline to 2 months, while maintaining excellent tolerability across different hair loss conditions [14].
Materials and Methods
Study Design and Setting:
This prospective observational study was conducted at Dr.
Vimala Manne’s dermatology clinic from January-December 2024,
evaluating the clinical efficacy and safety of Verilich serum in
enhancing treatment outcomes for chronic telogen effluvium, male
pattern hair loss, and female pattern hair loss. All procedures followed
the Declaration of Helsinki and good clinical practice guidelines [15].Rationale for Verilich Serum Selection:
Verilich serum was selected based on preliminary clinical
observations suggesting potential benefit in hair loss management.
As a commercially available topical formulation in India, it offered an
opportunity to systematically evaluate its role within comprehensive
treatment protocols for diverse hair loss conditions. The decision to
incorporate nutritional supplementation alongside Verilich serum
reflects contemporary clinical practice, where multimodal approaches
address the multifactorial nature of hair loss disorders.Verilich serum is a proprietary topical formulation containing [peptides, biotin, plant extracts, amino acids, vitamins]. While precise mechanisms were not investigated in this study, the formulation theoretically targets multiple pathways:
• Growth factor modulation supporting anagen phase maintenance
• Scalp microcirculation enhancement promoting follicular nutrition
• Anti-inflammatory effects beneficial in telogen effluvium
• Antioxidant properties protecting follicles from oxidative stress
Patient Selection:
Inclusion Criteria: Age 18-50 years; clinical diagnosis confirmed
by dermoscopy/trichoscopy of chronic telogen effluvium (≥6 months
shedding, positive hair pull test >6 hairs), male pattern hair loss
(Hamilton-Norwood classification), or female pattern hair loss
(Ludwig classification); willingness to comply with protocol; written
informed consent.Exclusion Criteria:Alopecia areata, scarring alopecias, active
scalp infections, pregnancy/lactation, hair growth treatments within
3 months, severe systemic illness, known hypersensitivity to study
components.
Study Population:
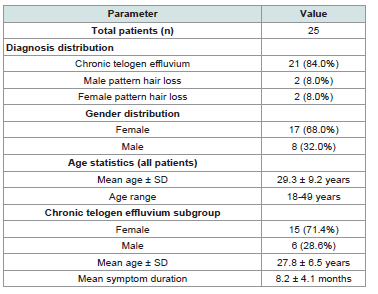
Twenty-five patients completed the study:21 with chronic
telogen effluvium (15 females, 6 males; mean age 27.8 years), 2 with
male pattern hair loss (mean age 38 years), and 2 with female pattern
hair loss (mean age 41.5 years).Treatment Protocol:
Verilich Serum:Daily topical application of 1mL to affected
scalp areas on clean, dry scalp with gentle massage for 2-3 minutes;
no washing for minimum 4 hours post-application.Nutritional Supplementation: All patients received iron supplements (individualized dosing), vitamin D3 60,000 IU weekly for 3 months, and methylcobalamin 500mcg daily for 3 months. Patients with severe anemia (hemoglobin <10 g/dL, ferritin <10 ng/ mL) received IV iron (Orafer-FCM 500mL) bi-weekly for 2 cycles.
Condition-Specific Therapy:Male pattern hair loss patients received finasteride 1mg or oral minoxidil 2.5mg daily. Female pattern hair loss patients received topical minoxidil 2-5% or hormonal therapy as appropriate.
Outcome Measurements:
Primary Endpoint:Time to clinical improvement defined
as >50% reduction in daily hair shedding (patient-reported) and
negative hair pull test (<3 hairs extracted from three scalp areas:
frontal, vertex, occipital).Secondary Endpoints:Safety/tolerability assessment, adverse event monitoring, patient satisfaction, photographic documentation.
Clinical Assessment:
Baseline:Medical history, trichoscopy (10x-70x magnification),
hair pull test (three scalp areas), standardized photography, laboratory
investigations (CBC, ferritin, vitamin D3, B12, thyroid function).Follow-up:Week 4 (initial response), Month 2 (primary endpoint), Month 3 (final assessment), Month 6 (pattern hair loss long-term follow-up).
Safety Monitoring
Systematic monitoring for local reactions (erythema, scaling, pruritus), systemic side effects (headache, dizziness, GI symptoms), contact sensitization, and drug interactions. All adverse events documented and graded for severity and relationship to treatment.
Statistical Analysis
Descriptive statistics summarized demographics and clinical characteristics. Treatment duration categorized as fast response (≤2 months) versus standard response (≥3 months). Comparison between chronic telogen effluvium and pattern hair loss groups using chisquare test (categorical variables) and independent t-test (continuous variables). P<0.05 considered statistically significant.
Quality Control
All assessments performed by the same dermatologist (Dr. Vimala Manne). Standardized photography protocol maintained (same camera, lighting, distance, angle). Regular dermoscopy equipment calibration. Patient compliance monitored through diary cards and product accountability.
Results
Patient Demographics and Baseline Characteristics:
Twenty-five patients with various hair loss conditions completed
the study protocol. The demographic profile [Table 1] demonstrates
a diverse patient population representative of typical dermatology
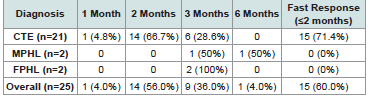
practice.Primary Efficacy Outcomes:
The primary efficacy endpoint demonstrated clinical
improvement across all diagnostic groups, with notable acceleration
in chronic telogen effluvium patients compared to conventional
therapy timelines [Table 2] .Key Findings:
• 71.4% of chronic telogen effluvium patients (15/21) achieved clinical improvement within 2 months, defined as >50% reduction in daily hair shedding and negative hair pull test (<3 hairs extracted from three scalp areas)
• All pattern hair loss patients responded within 3-6 months with reduced hair loss and improved hair density
• Overall fast response rate: 60% (15/25)
Progressive clinical improvement in chronic telogen effluvium patients followed a consistent temporal pattern. Initial response emerged at week 3-4 (14.3%, 3/21 with negative hair pull test). By month 2, 71.4% (15/21) achieved the primary endpoint with significant shedding reduction and negative hair pull test. All 21 patients (100%) achieved clinical improvement by month 3.
Subgroup analysis of chronic telogen effluvium patients revealed consistent response patterns across demographics: female (73.3%, 11/15) versus male (66.7%, 4/6) fast responders (p=0.89); no significant differences by age groups (p=0.98). Treatment response was independent of gender, age, or symptom duration.
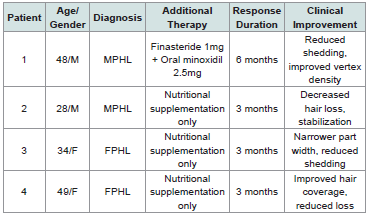
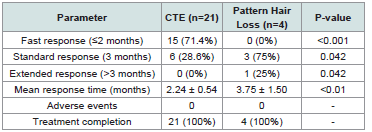
Pattern Hair Loss Treatment Outcomes:
Pattern hair loss patients demonstrated clinical improvement
within timeframes consistent with conventional therapy expectations
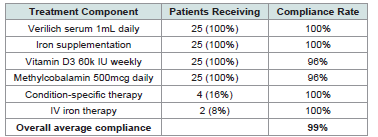
[Table 3] .Treatment Protocol Implementation and Safety:
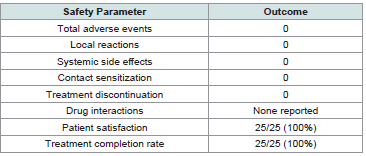
All patients successfully completed the comprehensive treatmentprotocol with excellent adherence [Table 4] .
The safety analysis demonstrated excellent tolerability across all
patient groups and diagnostic categories with zero adverse events
reported.
Comparative Analysis: CTE versus Pattern Hair Loss:
Statistical comparison between diagnostic groups revealed
significant differences in response patterns [Table 6].
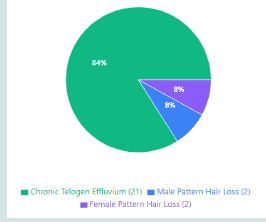
Figure 1:Patient distribution by diagnosis - pie chart showing CTE 84%,
MPHL 8%, FPHL 8%
Analysis of chronic telogen effluvium triggers revealed post-partum hair loss (23.8%, 5/21), iron deficiency/anemia (9.5%, 2/21), stress/lifestyle changes (4.8%, 1/21), and multiple/unspecified factors (61.9%, 13/21).
Analysis of chronic telogen effluvium triggers revealed post-partum hair loss (23.8%, 5/21), iron deficiency/anemia (9.5%, 2/21), stress/lifestyle changes (4.8%, 1/21), and multiple/unspecified factors (61.9%, 13/21).
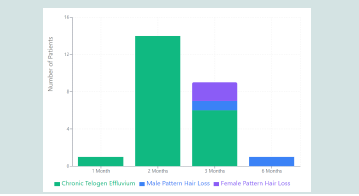
Clinical Response Characteristics:
Chronic telogen effluvium fast responders (≤2 months)
demonstrated progressive reduction in hair shedding beginning at
week 3-4 (reported by 90%), conversion to negative hair pull test
by month 2, improved hair shaft quality with reduced breakage,
and enhanced patient-reported quality of life. Pattern hair loss
patients showed gradual reduction in daily shedding over 3 months,
stabilization of hair loss progression, and visible density improvement
at affected areas.Two chronic telogen effluvium patients with severe anemia (hemoglobin <10 g/dL, ferritin <10 ng/mL) received additional IV iron therapy. Both achieved clinical improvement (one at 2 months, one at 3 months), demonstrating protocol efficacy even in complex cases requiring intensive nutritional rehabilitation.
Discussion
Principal Findings:
This prospective observational study demonstrates that Verilich
serum, when incorporated into comprehensive treatment protocols
for multiple hair loss conditions, contributes to favorable clinical
outcomes with differential response patterns depending on underlying
diagnosis. In chronic telogen effluvium, 71.4% achieved clinical
improvement within 2 months-characterized by >50% reduction
in daily hair shedding and negative hair pull test—compared to
conventional 3-6-month timelines [1,2]. Pattern hair loss patients
responded within expected timeframes (3-6 months) with excellent
tolerability across all diagnostic categories.
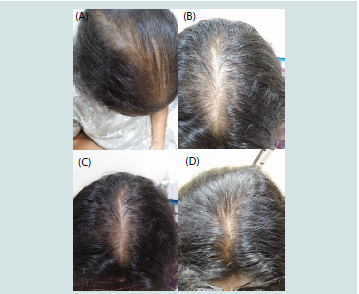
Figure 4:Clinical Documentation of Treatment Response in Chronic Telogen
Effluvium Patient Treated with Verilich Serum Adjunctive Therapy
(A) Baseline presentation showing diffuse hair thinning with widened central
part (measuring 2.8 cm) and increased scalp visibility. Patient: 32-year-old
female, 5 months post-partum, presenting with 8-month history of excessive
hair shedding (>150 hairs/day by patient count). Positive hair pull test (8-12
hairs extracted per site). Serum ferritin: 15 ng/mL.
(B) Month 2 assessment demonstrating improved hair density along central
part with reduced scalp visibility. Part width narrowed to 1.8 cm. Patient
reported >60% reduction in daily hair shedding (~50 hairs/day). Hair pull
test: negative (<2 hairs extracted per site). Patient achieved primary endpoint
criteria at this time point, representing the fast responder cohort (71.4% of
chronic telogen effluvium patients).
(C) Month 3 follow-up showing continued improvement in hair coverage and
density. Part width further reduced to 1.4 cm. Minimal daily hair shedding
reported (<30 hairs/day). Maintained negative hair pull test. Improved hair
shaft quality with reduced breakage noted on examination.
(D) Month 3 lateral view demonstrating overall improvement in hair volume
and coverage. Enhanced hair quality with visible increase in terminal hair
density at temporal and frontal regions. Patient satisfaction rating: 9/10.
Technical Details: All photographs obtained using standardized protocol
with identical camera settings (Canon EOS, 50mm lens, f/5.6, ISO 400),
lighting conditions (two 5000K LED panels positioned at 45° angles, 1 meter
distance), and patient positioning (seated, head tilted 15° forward for central
part views). Photographs taken at same time of day (10:00-11:00 AM) to
ensure consistent lighting. Room temperature maintained at 22°C, humidity
50%.
Patient Consent: Written informed consent obtained for clinical photography and publication. Patient confidentiality maintained through facial feature exclusion.
Clinical Significance: This case exemplifies the typical fast response pattern observed in 71.4% of chronic telogen effluvium patients, with achievement of objective clinical improvement criteria (>50% shedding reduction, negative hair pull test) by month 2. The progressive improvement from baseline through month 3 demonstrates the temporal dynamics of treatment response in chronic telogen effluvium.
Patient Consent: Written informed consent obtained for clinical photography and publication. Patient confidentiality maintained through facial feature exclusion.
Clinical Significance: This case exemplifies the typical fast response pattern observed in 71.4% of chronic telogen effluvium patients, with achievement of objective clinical improvement criteria (>50% shedding reduction, negative hair pull test) by month 2. The progressive improvement from baseline through month 3 demonstrates the temporal dynamics of treatment response in chronic telogen effluvium.
The demographic profile of our chronic telogen effluvium
population aligns with established epidemiological data: female
predominance (71.4%) and mean age of 27.8 years, consistent with
peak incidence in women of reproductive age [3]. The inclusion
of pattern hair loss patients (16% of cohort) provides preliminary
data on Verilich serum’s role within diverse hair loss management
strategies.
Comparative Analysis with Existing Therapies:
Traditional chronic telogen effluvium management focuseson nutritional supplementation, with clinical improvement
typically requiring 3-6 months [4]. Studies demonstrate that
iron supplementation alone requires extended periods to achieve
meaningful outcomes, often leading to poor compliance [5]. Our
findings suggest that comprehensive protocols incorporating Verilich
serum alongside nutritional supplementation may enhance treatment
outcomes in chronic telogen effluvium specifically.
The accelerated response represents meaningful clinical advantage. Achievement of objectively defined improvement (>50% shedding reduction, negative hair pulls test) within 2 months in 71.4% of patients addresses a critical gap in current management. Unlike topical minoxidil requiring 4-6 months with potential initial shedding exacerbation [6], our protocol demonstrated progressive improvement beginning at week 3-4 without worsening hair loss.
For pattern hair loss, our results align with conventional therapy timelines [7,8]. The 3-6-month response observed is consistent with standard minoxidil and finasteride expectations. However, the excellent safety profile with zero adverse events suggests potential advantages as part of long-term management strategies for conditions requiring chronic therapy.
The accelerated response represents meaningful clinical advantage. Achievement of objectively defined improvement (>50% shedding reduction, negative hair pulls test) within 2 months in 71.4% of patients addresses a critical gap in current management. Unlike topical minoxidil requiring 4-6 months with potential initial shedding exacerbation [6], our protocol demonstrated progressive improvement beginning at week 3-4 without worsening hair loss.
For pattern hair loss, our results align with conventional therapy timelines [7,8]. The 3-6-month response observed is consistent with standard minoxidil and finasteride expectations. However, the excellent safety profile with zero adverse events suggests potential advantages as part of long-term management strategies for conditions requiring chronic therapy.
Mechanistic Considerations:
The differential treatment response between chronic telogen
effluvium and pattern hair loss provides insights into potential
mechanisms through which comprehensive protocols including
Verilich serum may contribute to clinical outcomes. The accelerated
response in chronic telogen effluvium suggests the protocol may
particularly benefit conditions characterized by acute follicle
dysfunction and inflammatory processes, rather than exclusively
addressing chronic androgen-mediated miniaturization in pattern
hair loss.The consistent efficacy across different chronic telogen effluvium
demographic subgroups—independent of age, gender, or symptom
duration—suggests the treatment protocol may address fundamental
telogen effluvium pathophysiology rather than specific triggers. This
broad applicability could simplify clinical decision-making.
Safety Profile and Clinical Practicality:
The exceptional safety profile with zero adverse events across all
25 patients and diagnostic categories represents an important clinical
consideration. Traditional hair loss treatments can be associated
with tolerability issues: gastrointestinal disturbances from iron, scalp
irritation from minoxidil, sexual side effects from finasteride [9,10].
The absence of adverse reactions enhances clinical utility and patient
acceptability across diverse conditions.The excellent compatibility with concurrent therapies is particularly relevant for clinical practice. The ability to incorporate Verilich serum alongside iron supplements, vitamins, finasteride, minoxidil, and hormonal therapy without drug interactions represents practical clinical advantage, supporting integration into diverse treatment strategies.
Clinical Practice Implications:
For chronic telogen effluvium, the potential to achieve clinical
improvement in over 70% of patients within 2 months—with
objectively measurable outcomes—addresses a critical challenge:
patient frustration with slow response. Early improvement may
enhance patient confidence and improve long-term compliance with
comprehensive protocols.For pattern hair loss, while treatment duration remains consistent with conventional expectations, the excellent safety profile supports consideration of Verilich serum as a component of comprehensive strategies. This is particularly important given chronic therapy requirements where tolerability becomes critical for sustained compliance.
Study Limitations:
The observational design without control group limits definitive
attribution of accelerated chronic telogen effluvium response solely
to Verilich serum, as all patients received comprehensive nutritional
supplementation concurrently. The study reflects real-world practice
where multiple interventions are employed simultaneously, but
controlled studies would clarify specific contributions of individual
components.The small pattern hair loss sample size (n=4) limits generalizability for these conditions. Future studies should include larger pattern hair loss cohorts. Single-center design may limit generalizability, though demographic diversity of the chronic telogen effluvium cohort suggests broader applicability.
Long-term follow-up beyond 6 months would provide insights into response durability and optimal treatment duration. Randomized controlled trials with larger populations and separate treatment arms would strengthen evidence. Mechanistic studies investigating pathways through which Verilich serum may contribute to outcomes could inform future therapeutic optimization.
Conclusion
This study provides evidence that Verilich serum may play a
beneficial role within comprehensive management strategies for hair
loss conditions, with differential efficacy patterns across diagnoses. In
chronic telogen effluvium, the treatment protocol including Verilich
serum was associated with accelerated clinical improvement—
defined by objective criteria of >50% shedding reduction and negative
hair pull test—in 71.4% of patients within 2 months. Pattern hair loss
patients demonstrated improvement within expected timeframes.
The excellent safety profile with zero adverse events and high
treatment compliance support integration of Verilich serum into
comprehensive treatment protocols, particularly for chronic telogen
effluvium where accelerated response was observed.
References
2. Rebora A (2019) Telogen effluvium: a comprehensive review. Clin Cosmet Investig Dermatol 12:583-590.