Journal of Clinical and Investigative Dermatology
Download PDF
· Randomized and non-randomized clinical trials
· Study in patients diagnosed with vitiligo by any diagnostic methods
· Intervention of oral vitamin D supplementation with or without other treatment modalities, compared to without oral vitamin D supplementation in the treatment of vitiligo patients
· Study with at least one of the following outcome measurements: Vitiligo Area Severity Index (VASI score) and serum vitamin D levels
· Duplicate publications
· Studies with insufficient data for pooling even after attempts of contacting the author/s
· Study published in languages the reviewers cannot translate
1. “vitiligo”
2. “oral vitamin D supplementation”
3. “serum 25(OH)D” or “serum 25-hydroxy vitamin D”
4. “VASI scoring” or “Vitiligo Area Severity Index scoring”
5. “trial” OR “clinical trial”
These terms were used into accessible electronic databases, including PubMed Central, Cochrane Library, Google Scholar, Embase, Science Direct, Clinical Key, eMedicine, Wiley Online Library, and Herdin. Additionally, the reference lists of all collected studies pertinent to the research were reviewed.
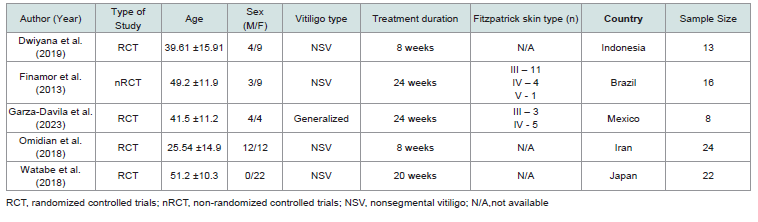
The selected studies were either randomized controlled trials (RCTs) or non-randomized controlled trials (nRCTs) conducted within the last 11 years. The sample sizes of these studies ranged from 8 to 24 participants, with age ranges from 25 to 51 years. These trials involved patients with either generalized or non-segmental vitiligo, without specifying the affected areas of the body. The studies were conducted in various countries: Indonesia, Brazil, Mexico, Iran, and Japan. Although all five studies reported serum vitamin D levels of the patients, only two included data on the Vitiligo Area Scoring Index (VASI) scores. The characteristics of the trials are summarized in [Table 1] , and details on the experimental and control groups are summarized in [Table 2] .
All five included RCTs involved vitamin D supplementation, administered orally at dosages of either 5,000 IU or 35,000 IU once daily, or 50,000 IU once every two weeks and measurement of serum 25(OH)D3 levels pre and post-intervention (Table 2). Additionally, three of these studies incorporated narrowband ultraviolet B (NBUVB) therapy as part of the treatment. One study permitted patients to continue using their current topical glucocorticoids or topical tacrolimus.
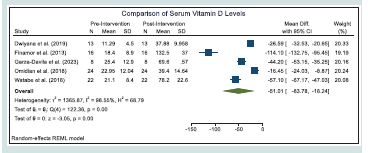
Based on the result of the statistical test, we find that the overall mean difference in the serum vitamin D levels of the patients before and after the vitamin D supplementation to be statistically significant. As presented in the [Table 4] , we find significant increase in the serum vitamin D levels of the patients after vitamin D supplementation across all studies. [Figure 2] shows the forest plot of the meta-analysis conducted on the changes in serum vitamin D levels of the patients
Based on the homogeneity tests based on the Q statistic indicated a statistically significant presence of heterogeneity in studies on changes in serum vitamin D levels but not in studies on changes in VASI scores. [Table 6] summarizes the different heterogeneity measures from the meta-analyses.
The result of this study coincides with the In vitro studies done by Tomita et. al, showed that vitamin D3 increases the tyrosinase content of cultured melanocytes. [4] This was further elaborated by the study of Watabe et al. wherein they observed an increase in L-3,4-dihydroxyphenylalanine-positive (DOPA-positive) cells following treatment with 1,25(OH) 2D3 in primary neural crest cell cultures.[15] These results suggest that 1,25(OH) 2D3 may promote the maturation of early melanocyte precursors. Moreover, the study of Karaguzel et. al [14] demonstrated that combining oral vitamin D with topical tacrolimus in pediatric patients with vitiligo is more effective at achieving repigmentation compared to using topical tacrolimus alone. Collectively, these studies suggest that it is plausible to consider vitamin D supplementation as a potential therapy for autoimmune diseases like vitiligo.
Research Article
Efficacy of Oral Vitamin D Supplementation on the Serum Vitamin D Levels and Disease Severity of Vitiligo Patients: A Systematic Review of Randomized Controlled Trials
Cadacio KAD, Rescober-Valencia C and King-Ismael D*
Department of Dermatology Resident Jose R. Reyes Memorial Medical Center, Manila, Philippines
*Address for Correspondence:Karen Andrea D. Cadacio, Department of Dermatology Resident
Jose R. Reyes Memorial Medical Center, Manila, Philippines Email Id: kdcadacio@gmail.com
Submission: 09 October, 2025
Accepted: 29 October, 2025
Published: 31 October, 2025
Copyright: © 2025 Cadacio KAD, et al. This is an open access
article distributed under the Creative Commons Attribution
License, which permits unrestricted use, distribution, and
reproduction in any medium, provided the original work is
properly cited.
Keywords:Vitiligo; Oral Vitamin D Supplementation; Systematic
Review; Serum Calcium Levels; VASI Score
Abstract
Introduction: Vitiligo is an autoimmune skin condition where CD8+
T cells target and destroy melanocytes, leading to depigmented
patches. Vitamin D has been found to play a key role in melanogenesis
by stimulating melanocyte activity. Vitamin D deficiency is linked to
autoimmune diseases like vitiligo, making vitamin D supplementation a
potential therapeutic approach.
Objectives: This study aims to determine the efficacy of oral vitamin D supplementation on serum vitamin D levels and disease severity in vitiligo patients through an in-depth systematic review of randomized controlled trials.
Results: A total of five randomized controlled trials (RCTs) were included in the study. All five studies reported serum vitamin D levels, while only two provided data on Vitiligo Area Scoring Index (VASI) scores. The overall mean difference in serum vitamin D levels before and after vitamin D supplementation was found to be statistically significant, with a consistent increase across all studies. Similarly, the mean difference in VASI scores was also statistically significant, showing a notable decrease in vitiligo severity after supplementation in the two studies. Homogeneity tests based on the Q statistic indicated significant heterogeneity and publication bias was identified in studies related to changes in serum vitamin D levels, but not in those measuring VASI scores.
Conclusion: Oral vitamin D supplementation significantly increases serum vitamin D levels and may improve vitiligo severity. Studies with larger sample sizes and standardized methodologies are needed to further confirm these findings.
Objectives: This study aims to determine the efficacy of oral vitamin D supplementation on serum vitamin D levels and disease severity in vitiligo patients through an in-depth systematic review of randomized controlled trials.
Results: A total of five randomized controlled trials (RCTs) were included in the study. All five studies reported serum vitamin D levels, while only two provided data on Vitiligo Area Scoring Index (VASI) scores. The overall mean difference in serum vitamin D levels before and after vitamin D supplementation was found to be statistically significant, with a consistent increase across all studies. Similarly, the mean difference in VASI scores was also statistically significant, showing a notable decrease in vitiligo severity after supplementation in the two studies. Homogeneity tests based on the Q statistic indicated significant heterogeneity and publication bias was identified in studies related to changes in serum vitamin D levels, but not in those measuring VASI scores.
Conclusion: Oral vitamin D supplementation significantly increases serum vitamin D levels and may improve vitiligo severity. Studies with larger sample sizes and standardized methodologies are needed to further confirm these findings.
Introduction
Vitiligo is a skin condition where melanocytes progressively
decrease, leading to well-defined milky-white macules and/or patches
on the skin, which can sometimes be accompanied by the loss of
pigmentation in hair, known as poliosis [1]. Varying prevalence rates
in different populations may be attributed to genetic or environmental
factors. Moreover, it is possible that social and cultural stigmas play a
role in the prevalence of vitiligo. Given these complexities, it is often
stated that the global prevalence of the disease is roughly consistent,
estimated to be around 0.5% to 1%.[1]
Vitiligo usually starts before the third decade of life. It tends to occur early in the segmental variant of vitiligo, which affects only one side of the body. There is no gender predilection with regards to its demographics; however, it has been that females seek treatment more frequently than males.[1]
Vitiligo usually starts before the third decade of life. It tends to occur early in the segmental variant of vitiligo, which affects only one side of the body. There is no gender predilection with regards to its demographics; however, it has been that females seek treatment more frequently than males.[1]
Vitiligo typically presents as painless, white patches with clear
edges that glow under a Wood’s lamp. These patches can appear
anywhere on the body, often symmetrically. While the condition
can begin in any areas, it frequently starts on the face, hands, feet,
or genital regions. Several clinical patterns exist, such as acrofacial,
mucosal, generalized, universal, mixed, and rare forms, but
distinguishing between them can be challenging due to frequent
overlaps or evolution. [1]
Vitiligo is a skin autoimmune condition where CD8+ T cells
attack and destroy melanocytes, leading to areas with no pigment
production. The cause is still a subject of debate however, it is now
understood that melanocytes in vitiligo patients are abnormal and
more susceptible to cellular stress processes, such as oxidative stress
and abnormal melanogenesis. This leads to the release of reactive
oxygen species and triggers the unfolded protein response, which,
in turn, prompts melanocytes to release signaling molecules that act
as danger signals, alerting the innate immune system. This, in turn,
activates and attracts adaptive immune CD8+ T cells to the skin,
where they identify and eliminate the abnormal melanocytes.[1]
Vitamin D is produced in the skin through a photochemical
process when the skin is exposed to sunlight containing ultraviolet B
(UVB) rays. During this reaction, previtamin D is transformed into
vitamin D. Vitamin D exists in two primary forms: cholecalciferol
and ergocalciferol. These forms can be acquired through dietary
sources. Ergocalciferol, known as vitamin D2, is naturally found in
fungi and yeast, while cholecalciferol, also known as vitamin D3, is
present in animal-derived foods (herring and mackerel).[2]
Given the well-established fact that calciferols can be
photochemically converted from ergosterol and 7-dehydrocholesterol
(pro-vitamin D3) through exposure to ultraviolet B (UVB) irradiation,
it was suggested that cholecalciferol could have a role in the natural
process of melanogenesis induced by sunlight in human skin. [3] This
was further proven by the Tomita et. al, saying that cholecalciferol,
or vitamin D, has a stimulating impact on melanocytes, specifically
in the context of photoinduced skin pigmentation. This includes the
initiation of tyrosinase synthesis, enlargement of cell size, and the
extension of cell dendrites, which facilitate the transfer of melanin
granules to nearby keratinocytes in the skin.[4]
Low vitamin D levels have been linked to vitiligo, an autoimmune
condition. Some experts believe that vitamin D deficiency might even
trigger autoimmune diseases. Therefore, vitamin D supplements
could potentially be used to treat vitiligo.
The synthesis of vitamin D occurs in epidermis from the precursor molecule 7-dehydrocholesterol (provitamin D3) to previtamin D3 by ultraviolet B (UVB) radiation [5]. Previtamin D3 next converted into vitamin D3 (cholecalciferol) through spontaneous, temperature dependent isomerization. Once in the circulation, vitamin D is transformed into 25-hydroxyvitamin D (25- (OH)D3 by hepatic hydroxylase enzyme The amount of circulating 25(OH)D3 in the blood is a good indicator of overall vitamin D status and can help diagnose deficiency. Getting enough sunlight and consuming vitamin D-rich foods are key to maintaining healthy vitamin D levels. However, the effectiveness of vitamin D supplements depends on a person’s initial vitamin D levels, so blood tests are recommended after starting treatment.
This study aims to determine the efficacy of oral vitamin D supplementation on serum vitamin D levels and disease severity in vitiligo patients. It reviewed randomized and non-randomized controlled trials assessing serum 25(OH)D3 levels and/or VASI scores in vitiligo patients to evaluate the potential benefits of vitamin D supplementation for this condition.
The synthesis of vitamin D occurs in epidermis from the precursor molecule 7-dehydrocholesterol (provitamin D3) to previtamin D3 by ultraviolet B (UVB) radiation [5]. Previtamin D3 next converted into vitamin D3 (cholecalciferol) through spontaneous, temperature dependent isomerization. Once in the circulation, vitamin D is transformed into 25-hydroxyvitamin D (25- (OH)D3 by hepatic hydroxylase enzyme The amount of circulating 25(OH)D3 in the blood is a good indicator of overall vitamin D status and can help diagnose deficiency. Getting enough sunlight and consuming vitamin D-rich foods are key to maintaining healthy vitamin D levels. However, the effectiveness of vitamin D supplements depends on a person’s initial vitamin D levels, so blood tests are recommended after starting treatment.
This study aims to determine the efficacy of oral vitamin D supplementation on serum vitamin D levels and disease severity in vitiligo patients. It reviewed randomized and non-randomized controlled trials assessing serum 25(OH)D3 levels and/or VASI scores in vitiligo patients to evaluate the potential benefits of vitamin D supplementation for this condition.
Objectives of the Study
The main objective of this study is to determine the efficacy of
oral vitamin D supplementation on the serum vitamin D levels and
disease severity of vitiligo patients.
The specific objectives are:
1. To conduct a thorough and in-depth review of the effectiveness in the treatment of vitiligo and in serum levels of vitamin D in response to oral vitamin D supplementation
2. To pool the mean differences and statistically compare serum vitamin D levels in vitiligo patients given oral vitamin D supplementation
The specific objectives are:
1. To conduct a thorough and in-depth review of the effectiveness in the treatment of vitiligo and in serum levels of vitamin D in response to oral vitamin D supplementation
2. To pool the mean differences and statistically compare serum vitamin D levels in vitiligo patients given oral vitamin D supplementation
Methodology
Study Design:
This research is a quantitative systematic review on the efficacy
of oral vitamin D supplementation in vitiligo patient. This study
adhered to the guidelines outlined in the Preferred Reporting Items
for Systematic Reviews and Meta-Analysis (PRISMA).Inclusion and Exclusion criteria:
Inclusion Criteria· Randomized and non-randomized clinical trials
· Study in patients diagnosed with vitiligo by any diagnostic methods
· Intervention of oral vitamin D supplementation with or without other treatment modalities, compared to without oral vitamin D supplementation in the treatment of vitiligo patients
· Study with at least one of the following outcome measurements: Vitiligo Area Severity Index (VASI score) and serum vitamin D levels
Exclusion Criteria:
Non-human research (i.e., animal or cell biology research)· Duplicate publications
· Studies with insufficient data for pooling even after attempts of contacting the author/s
· Study published in languages the reviewers cannot translate
Data Sources and Strategy:
Keywords used for the literature search include the following:1. “vitiligo”
2. “oral vitamin D supplementation”
3. “serum 25(OH)D” or “serum 25-hydroxy vitamin D”
4. “VASI scoring” or “Vitiligo Area Severity Index scoring”
5. “trial” OR “clinical trial”
These terms were used into accessible electronic databases, including PubMed Central, Cochrane Library, Google Scholar, Embase, Science Direct, Clinical Key, eMedicine, Wiley Online Library, and Herdin. Additionally, the reference lists of all collected studies pertinent to the research were reviewed.
Data Collection:
The primary investigator selected and screened articles by the
information from the title and abstract, based on the above mentioned
inclusion and exclusion criteria. The primary investigator then
thoroughly read on the screened articles to gather data including the
author, publication, patient characteristics, sample size, intervention,
treatment protocol, outcome measures.Outcome Measures:
The primary outcomes collected in each journal article include:
1) serum vitamin D levels of vitiligo patients before and after
intervention, and/or 2) disease extent and severity using VASI
score. Serum 25-hydroxyvitamin D [25(OH)D] concentration is
considered the gold standard biomarker for vitamin D status, widely
used in clinical and epidemiologic research. An increase in serum
25(OH)D levels following supplementation represents improved
systemic vitamin D status. VASI score, on the other hand, is a
validated clinical tool that quantifies the extent and severity of vitiligo
lesions, directly correlating with patient burden and repigmentation
outcomes. Together, these outcome measures allow assessment
of both biochemical efficacy and clinical impact of oral vitamin D
supplementation.Statistical Analysis Plan:
All statistical analyses in this systematic review were performed
using Revman 5.4.1, following the PRISMA guidelines. Difference
between the serum vitamin D levels in response to the oral vitamin D
supplementation were analyzed. The results of each RCT were plotted
into Revman to create a forest plot to derive the resulting direct effects
and the studies significance. To ensure quality of the RCT, publication
bias analysis and test of heterogeneity were employed.Quality Assessment:
Studies were independently reviewed and assessed using the JBI
Critical Appraisal Tool for Randomized Controlled Trials. The JBI
Critical Appraisal Tool [6] for Randomized Controlled Trials is a
13-item checklist that provide a descriptive assessment of potential
studies to be included or excluded in the systematic review or metaanalysis.
Unlike traditional assessment tools, there is no threshold
which determines whether a study is to be included; rather, the
assessment lies on the judgement of the researchers.Results
Study Characteristics:
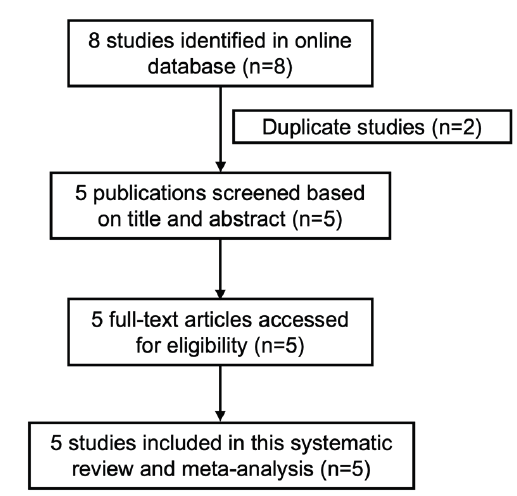
A total of 8 articles were collected by searching PubMed Central,
Cochrane Library, Google Scholar, Embase, Science Direct, Clinical
Key, eMedicine, Wiley Online Library, and Herdin. After removing 2
duplicate studies and 1 prospective cohort study, 5 articles remained
for screening based on the titles and abstracts. All 5 studies were
available and read in full. These 5 studies were included for the
systematic review and meta-analysis. [Figure 1]The selected studies were either randomized controlled trials (RCTs) or non-randomized controlled trials (nRCTs) conducted within the last 11 years. The sample sizes of these studies ranged from 8 to 24 participants, with age ranges from 25 to 51 years. These trials involved patients with either generalized or non-segmental vitiligo, without specifying the affected areas of the body. The studies were conducted in various countries: Indonesia, Brazil, Mexico, Iran, and Japan. Although all five studies reported serum vitamin D levels of the patients, only two included data on the Vitiligo Area Scoring Index (VASI) scores. The characteristics of the trials are summarized in [Table 1] , and details on the experimental and control groups are summarized in [Table 2] .
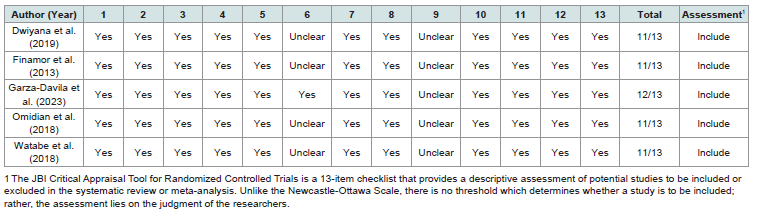
Risk of Bias:
The risk of bias assessment for randomized controlled trials
(RCTs) was evaluated using the JBI Critical Appraisal Tool. Based
on the researchers’ assessments, the studies by Dwiyana et al. (2019)
[5], Finamor et al. [7]. Garza-Davila et al. (2023) [8], Omidian et al. (2018) [9], and Watabe et al. (2018) [10] were included in the
systematic review and meta-analysis. Four out of the five studies
were randomized; however, only one detailed the randomization
procedure and allocation concealment. One study lacked a control
group, as all subjects underwent the intervention. Among the two
studies that included the Vitiligo Area Scoring Index (VASI) as
an outcome measure, only one specified that the assessment was
conducted by three blinded board-certified dermatologists. Detailed
information regarding the risk of bias is provided in [Table 3].Response to Therapy:
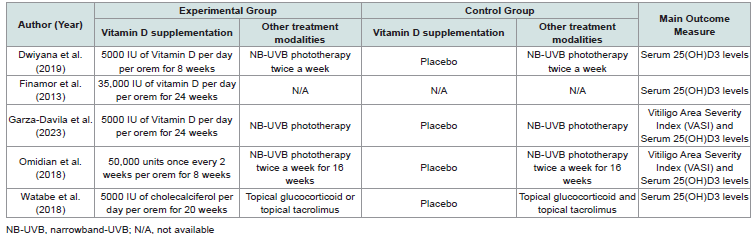
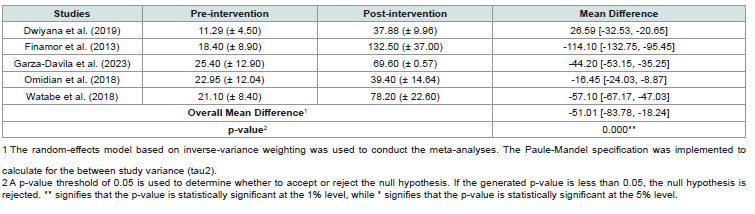
Serum 25(OH)D3 LevelsAll five included RCTs involved vitamin D supplementation, administered orally at dosages of either 5,000 IU or 35,000 IU once daily, or 50,000 IU once every two weeks and measurement of serum 25(OH)D3 levels pre and post-intervention (Table 2). Additionally, three of these studies incorporated narrowband ultraviolet B (NBUVB) therapy as part of the treatment. One study permitted patients to continue using their current topical glucocorticoids or topical tacrolimus.
Based on the result of the statistical test, we find that the overall mean difference in the serum vitamin D levels of the patients before and after the vitamin D supplementation to be statistically significant. As presented in the [Table 4] , we find significant increase in the serum vitamin D levels of the patients after vitamin D supplementation across all studies. [Figure 2] shows the forest plot of the meta-analysis conducted on the changes in serum vitamin D levels of the patients
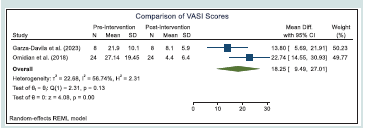
Vitiligo Area Severity Index (VASI):
Only two of the five studies included in this review used the
Vitiligo Area Scoring Index (VASI) as an outcome measure
[Table 2]. In these studies, the subjects underwent narrowband ultraviolet
B (NB-UVB) therapy, while the intervention group received either
5,000 IU of vitamin D daily or 50,000 IU once every two weeks as
supplementation. Table 5 summarizes the meta-analysis results
on the changes in VASI scores of the patients after vitamin D
supplementation.Based on the result of the statistical test, we find that the overall
mean difference in the VASI scores of the patients before and after the
vitamin D supplementation to be statistically significant. As presented
in the table, we find significant decrease in the VASI scores of the
patients after vitamin D supplementation across the two studies.
[Figure 3] shows the forest plot of the meta-analysis conducted on
the changes in VASI scores of the patients.
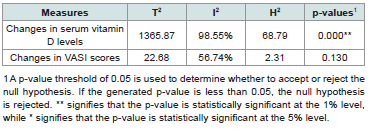
Measures of Heterogeneity and Publication Bias:
Measures of HeterogeneityBased on the homogeneity tests based on the Q statistic indicated a statistically significant presence of heterogeneity in studies on changes in serum vitamin D levels but not in studies on changes in VASI scores. [Table 6] summarizes the different heterogeneity measures from the meta-analyses.
Publication Bias:
We find a presence of publication bias for studies on changes in
serum vitamin D levels but not in studies on changes in VASI scores.
[Table 7] summarizes the results of Egger’s test for publication bias.Limitations of the Study:
Clinical trials exhibit variability in outcome measures, followup
periods, intervention periods. Fitzpatrick skin types, diets,
sun exposure, and other factors that may affect the results of each
study. Additionally, the clinical trials included in this study differ
in the dosing regimens of vitamin D supplementation, and some
incorporated additional interventions alongside oral vitamin D
supplementation, which may further influence the results. Limitations
of this study include small sample sizes, and the inclusion of nonrandomized
trials, all of which may impact the generalizability and
reliability of the findings.Discussion
Serum 25(OH)D3 Levels:
This systematic review revealed a statistically significant increase
in serum 25(OH)D levels and a concomitant improvement in VASI
scores following oral vitamin D supplementation in patients with
vitiligo. Forest plot analysis of serum 25(OH)D levels demonstrated
a consistent post-intervention elevation across studies. However,substantial heterogeneity was observed, suggesting potential
variations in treatment response among participants.
A closer examination of the included studies indicates that the heterogeneity might be attributed to differing intervention protocols. While Finamor et al [7]. Exclusively employed oral vitamin D supplementation, the remaining studies incorporated additional therapies such as topical glucocorticoids, tacrolimus, or narrowband ultraviolet B (NB-UVB). Furthermore, variations in vitamin D dosage and treatment duration across studies could also contribute to the observed heterogeneity. Across trials, supplementation was administered at 5,000 IU/day, 35,000 IU/day, or 50,000 IU once every two weeks. These high-dose protocols were designed for research purposes and differ from standard clinical recommendations. According to the Endocrine Society guidelines, adults at risk of deficiency typically require 1,500–2,000 IU/day for maintenance, with higher doses only under supervision. To ensure safety, serum 25(OH) D and calcium are generally monitored every 8-12 weeks, particularly with high-dose or bolus regimens. In the reviewed trials, laboratory testing was consistently performed at baseline and post-intervention, and some incorporated mid-treatment monitoring, ensuring both efficacy and safety surveillance.
A closer examination of the included studies indicates that the heterogeneity might be attributed to differing intervention protocols. While Finamor et al [7]. Exclusively employed oral vitamin D supplementation, the remaining studies incorporated additional therapies such as topical glucocorticoids, tacrolimus, or narrowband ultraviolet B (NB-UVB). Furthermore, variations in vitamin D dosage and treatment duration across studies could also contribute to the observed heterogeneity. Across trials, supplementation was administered at 5,000 IU/day, 35,000 IU/day, or 50,000 IU once every two weeks. These high-dose protocols were designed for research purposes and differ from standard clinical recommendations. According to the Endocrine Society guidelines, adults at risk of deficiency typically require 1,500–2,000 IU/day for maintenance, with higher doses only under supervision. To ensure safety, serum 25(OH) D and calcium are generally monitored every 8-12 weeks, particularly with high-dose or bolus regimens. In the reviewed trials, laboratory testing was consistently performed at baseline and post-intervention, and some incorporated mid-treatment monitoring, ensuring both efficacy and safety surveillance.
Several studies have correlated the relationship between serum
vitamin D levels and predisposition to or severity of vitiligo. A metaanalysis
of observational studies conducted by Upala et al. has shown
a correlation between lower serum levels of 25-hydroxyvitamin D
and vitiligo [11] similar to our study’s results. However, it remains
uncertain whether this associated with vitiligo, similar to its role in
other autoimmune diseases. Similarly, Saleh et al.’s study found that
patients having both vitiligo and autoimmune diseases had lower
serum levels of 25(OH)D compared to vitiligo patients without
autoimmune conditions, although this difference was not statistically
significant. [12] This study also concluded that age, duration of
vitiligo, affected body surface area has no statistically significant
correlations with the serum 25(OH)D levels of patients.
On the other hand, studies by Khurum et al [13] and Karaguzel et al [14] to determine the level of serum vitamin D in adult13 and pediatric14 vitiligo patients compared to control’s reveal that there is no significant difference in vitamin D serum levels between individuals with vitiligo and those without. However, the deficiency in 25(OH)D levels within the various subgroups of vitiligo patients could be associated with factors such as younger age, male gender, a shorter duration of vitiligo, and the absence of phototherapy use.[13]
On the other hand, studies by Khurum et al [13] and Karaguzel et al [14] to determine the level of serum vitamin D in adult13 and pediatric14 vitiligo patients compared to control’s reveal that there is no significant difference in vitamin D serum levels between individuals with vitiligo and those without. However, the deficiency in 25(OH)D levels within the various subgroups of vitiligo patients could be associated with factors such as younger age, male gender, a shorter duration of vitiligo, and the absence of phototherapy use.[13]
Environmental and Geographic Factors:
Environmental and geographic factors also influenced baseline
vitamin D levels. Endogenous synthesis varies depending on latitude,
climate, and sun exposure. For example, patients in equatorial regions
such as Indonesia and Brazil may start with higher vitamin D status
compared to patients in Japan, were lower UV indices and seasonal
variation limit synthesis. Dietary intake adds further variability. These
environmental and nutritional differences should be accounted for in
future trials to reduce confounding.Vitiligo Area Severity Index:
The forest plot analysis of VASI scores demonstrated a significant
reduction in vitiligo severity following vitamin D supplementation
across both included studies. Despite the clear overall benefit,
moderate heterogeneity was observed, suggesting potential variations
in treatment response among patient populations or study conditions.
Although both studies incorporated NB-UVB therapy, differences
in vitamin D dosage regimens may have influenced the observed
heterogeneity. Moreover, it can be inferred that bolus dosing of
vitamin D presented with better VASI scores.The result of this study coincides with the In vitro studies done by Tomita et. al, showed that vitamin D3 increases the tyrosinase content of cultured melanocytes. [4] This was further elaborated by the study of Watabe et al. wherein they observed an increase in L-3,4-dihydroxyphenylalanine-positive (DOPA-positive) cells following treatment with 1,25(OH) 2D3 in primary neural crest cell cultures.[15] These results suggest that 1,25(OH) 2D3 may promote the maturation of early melanocyte precursors. Moreover, the study of Karaguzel et. al [14] demonstrated that combining oral vitamin D with topical tacrolimus in pediatric patients with vitiligo is more effective at achieving repigmentation compared to using topical tacrolimus alone. Collectively, these studies suggest that it is plausible to consider vitamin D supplementation as a potential therapy for autoimmune diseases like vitiligo.
Conclusion and Recommendations
In conclusion this systematic review suggests that oral vitamin D
supplementation shows statistically significant increase in the serum
vitamin D levels in vitiligo patients. It also shows improvement on
vitiligo severity, as measured by the VASI scoring system. The high
heterogeneity of the studies included in this review should be taken
into consideration. Further comprehensive studies, including larger
sample size, more high-quality RCTs with similar methodologies
are needed to conduct a more objective analysis on the efficacy of
oral vitamin D supplementation on the serum vitamin D levels and
disease severity of vitiligo patients.