Journal of Clinical and Investigative Dermatology
Download PDF
Case Report
Mycosis Fungoides Presenting After Dupilumab Therapy: Case Report and Systematic Review
Visconti M1, Teklehaimanot F2, Tan V2 and Fivenson D1,3*
1Department of Dermatology, St Joseph Mercy Hospital Ann Arbor,
Ypsilanti, Michigan
2Michigan State School of Osteopathic Medicine, East Lansing, Michigan
3Fivenson Dermatology, 3200 W Liberty Rd, Suite C5, Ann Arbor, Michigan
Submission: 24 March, 2023
Accepted: 04 April, 2023
Published: 02 May, 2023
*Address for Correspondence
Fivenson D, Fivenson Dermatology, 3200 W Liberty Rd, Suite C5,
Ann Arbor, Michigan 48103; USA; Phone: 734-222-9630; E-mail:
dfivenson@fivensondermatology.com
Copyright: © 2023 Visconti M, et al. This is an open access article
distributed under the Creative Commons Attri-bution License,
which permits unrestricted use, distribution, and reproduction in
any medium, provided the original work is properly cited.
Keywords: Atopic dermatitis; Cutaneous T cell Lymphoma;
Cytokine; Mycosis Fungoides; Cutaneous T cell Lymphoma; IL-13, IL-4;
IL-13Rα1 and IL-13Rα2
Abstract
Introduction: Several reports have been made associating
dupilumab therapy for atopic dermatitis (AD) with the development of
mycosis fungoides (MF) and other cutaneous T cell lymphomas (CTCL)
Methods: A new case report and systematic review were
conducted to identify reports of MF/CTCL after minimum 6 weeks of
dupilumab therapy between January 2021 and March 2023.
Results: 28 patients from 18 publications (including our case) were
identified, averaging 17.4 years of AD duration with 18.5 weeks of
dupilumab therapy prior to MF/CTCL diagnosis. MF/CTCL presented
as 6 stage I, 4 stage II, 3 stage III, 4 stage IV, 3 Sezary syndrome, 6
large cell transformation, 2 peripheral T cell lymphoma (PTCL) and 1
with coexisting Hodgkin’s lymphoma. There were 13 females, 15 males,
and included 2 African-Americans, 4 Asians, and 22 Caucasians. 11
patients had initial improvement on dupilumab.
Conclusion: Clinical unmasking of MF/CTCL or de novo
lymphomagenesis as the mechanism in these patients is unknown.
Increased IL-13 and/or inhibition of reactive T cells through IL-4/IL-
13 blockade are possible mechanisms of action. Awareness of this
phenomenon during AD treatment and close follow-up and biopsy of
non-responders or those who develop new morphologies is important.
Introduction
Mycosis Fungoides (MF) is the most common subtype of
cutaneous T-cell lymphoma (CTCL) [1,2]. The disease is characterized
by patches, plaques, and tumors that can be intensely pruritic and
present in various sizes, shapes, and colors [2]. The cutaneous
findings of MF can mimic other common cutaneous diseases such
as atopic dermatitis, psoriasis, and parapsoriasis, making diagnosis
challenging, and often requiring serial biopsies [3].
While the etiology of MF remains unknown, various hypotheses
have been proposed which include genetic, environmental, infectious,
and autoimmune involvement [4,5]. Recently, there have been several
reports of MF diagnosed after the initiation of dupilumab therapy for
atopic dermatitis (AD) or other eczematous conditions [6-23]. In this
report, we present another case of AD developing stage IIB MF in the
ensuing months after starting dupilumab and a systematic review of
all published cases of this association, as well as the hypotheses for
how this transition may occur.
Methods
A systematic English language literature review using PubMed
and Google Scholar between January 2021 and March 2023, with
key words of (mycosis fungoides and dupilumab, cutaneous T cell
lymphoma and dupilumab, as well as descriptors of transformation,
progression and misdiagnosis) was conducted to identify reports of
the diagnosis of MF/CTCL in association with dupilumab treatment.
All relevant reports had full text review by the senior author. Inclusion
criteria included patients who were diagnosed with MF/CTCL
following treatment with dupilumab, any preexisting dermatosis
was included. Exclusion criteria included reports without histologic
confirmation of MF/CTCL and those with diagnosis after less than 3
doses (6 weeks of standard initial therapy) of dupilumab therapy. This
was done to exclude cases that could have been benign dermatoses
that were temporarily given dupilumab while diagnostic workup for
MF/CTCL was ongoing, as biologic transformation seems unlikely
in such a short time frame. Preexisting MF/CTCL reports that were
exposed to dupilumab were also excluded.
Data extracted included age, sex, AD duration, initial clinical and
histologic features, prior therapies, duration of dupilumab treatment,
MF/CTCL clinical presentation, MF histology, immunophenotyping,
TCR gene rearrangement, TNMB staging, and subsequent therapy.
Results
Case 1: A 77-year-old male with a 10-year history of AD,
presented to our clinic with worsening pruritus on the upper
trunk and buttocks. Skin biopsies previously had shown spongiotic
dermatitis with eosinophilic infiltrates. Prior treatments had included
narrow-band ultraviolet light phototherapy (NBUVB), high-potency
topical steroids (TS), and 17 weeks of dupilumab. Examination
revealed extensive annular, atrophic, erythematous, scaling, patches
and plaques which the patient noted had thickened since starting
dupilumab. Repeat skin biopsy revealed an acanthotic epidermis with
an atypical epidermotropic infiltrate of hyperchromatic-lymphocytes.
Immunophenotyping showed a predominant T cell infiltrate
that was CD2+, CD4+, CD1a-, CD3-, CD5-, CD8-, CD7-. T cell
receptor (TCR) gamma and beta gene rearrangements were positive.
Peripheral blood immunophenotyping, TCR, and PET scan revealed
no systemic involvement. The patient discontinued dupilumab and
started psoralen plus ultraviolet A photochemotherapy (PUVA)
treatments twice weekly. Improvement in disease extent and lesion
thickness was noted within 8 weeks and after 30 treatments the
patient was almost clear.
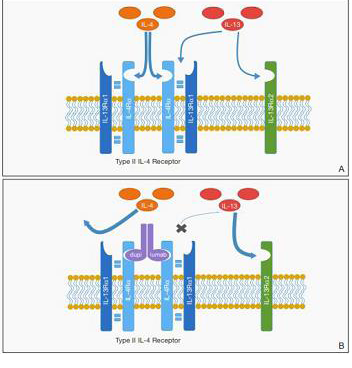
Figure 1: Proposed mechanism for promotion of cutaneous T-cell lymphoma
(CTCL) by dupilumab. A, IL-4 and IL-13 bind to the type II IL-4 receptor (IL-
4R). IL-13 also may bind at a distinct IL-13 receptor α2 (IL-13Rα2) site. B,
Following blockade of the type II IL-4R, reduced binding at IL-4R may result
in increased free IL-13 available for binding at the IL-13Rα2 site, which may
have a role in promoting CTCL proliferation. Reprinted with permission from
Hollins LC, et al, from Cutis.2020 August; 106: E8-E11. ©2020, Frontline
Medical Communications Inc.
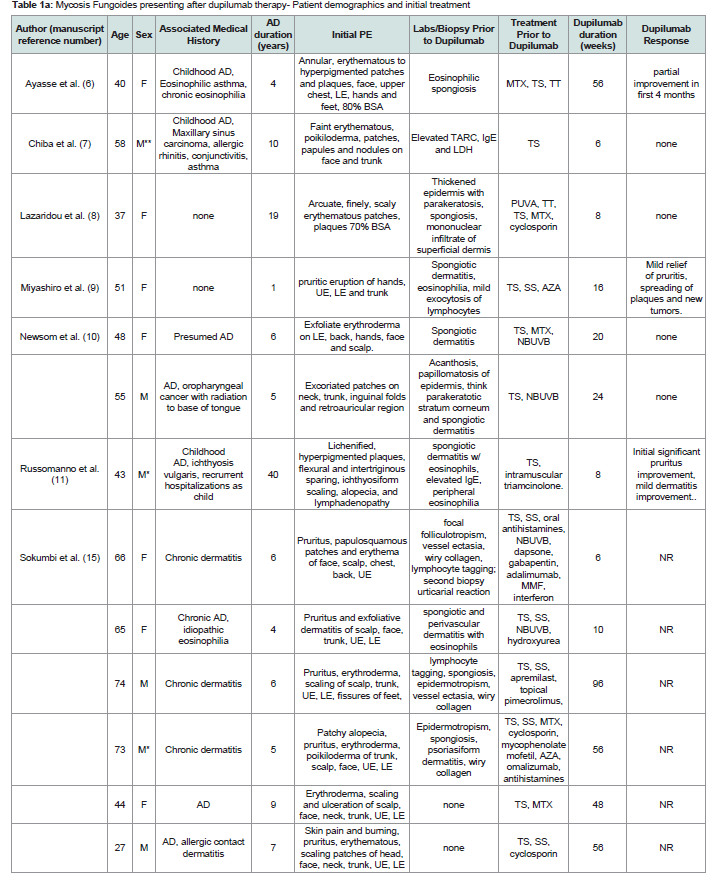
Patient demographics, clinical and histology before dupilumab therapy:
19 studies were included, totaling 28 patients included in our
series. 9 patients excluded after review of the publications due to
insufficient length of dupilumab exposure (n=3) or its use reported in
preexisting MF/CTCL subjects in the study (n=6)The demographics of the patients included in this study are
summarized in [Table 1]. There were 15 males and 13 females. There
were 22 Caucasian, 2 African American and 4 Asians with an age range
of 27-77 years and a mean of 55 years. Duration of disease ranged
from 1 to 58 years, mean= 17.4. Patients starting dupilumab therapy
had a history of AD or presumed AD although several did not report
skin biopsy findings pretreatment [Table 1] , with associated diseases
including IgE mediated disorders (asthma, allergic rhinitis, idiopathic
eosinophilia, and conjunctivitis) (n=5), and single cases each, allergic
contact dermatitis, maxillary sinus carcinoma, oropharyngeal cancer,
and ichthyosis vulgaris.
Clinical presentation and distribution of the skin eruption was
widespread with reports of extensive disease (>70% body surface
area-BSA) or exfoliative erythroderma prior to starting dupilumab
in 18/23 cases that described disease extent prior to dupilumab
therapy. Pruritus was dominant in most cases with morphologies
described including prototypical AD (lichenified and excoriated
flexural patches and plaques), urticarial, palmoplantar dermatitis,
ichthyosiform xerosis, blepharitis and conjunctivitis.
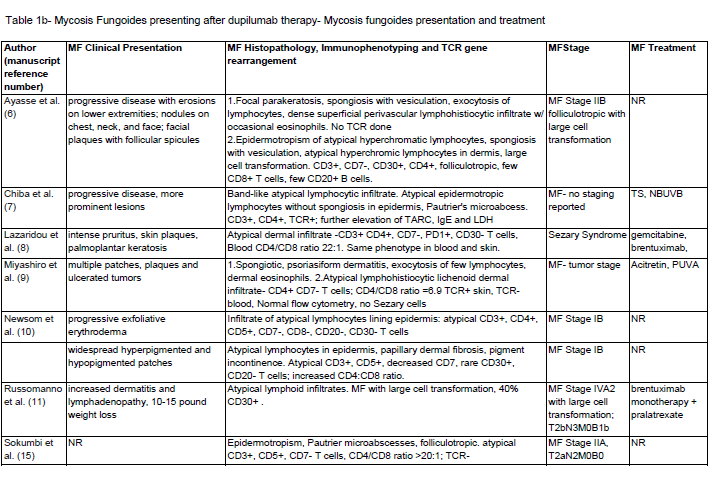
Post-dupilumab clinical and histologic findings:
Clinical and histologic features that changed after dupilumab
treatment are also summarized in [Table 1] . The mean duration of
therapy was 18.5 weeks prior to MF/CTCL diagnosis. There were 2
reports of complete clearing and 8 of partial improvement of symptoms
while on dupilumab. But eventual worsening and associated with
the progression of lesions occurred in all reports. Some describe the
development of secondary lesions including thicker plaques, nodules,
erosions, exfoliative erythroderma, and ulcerating tumors.The majority of patients had CD3+, CD4+, CD7-, CD8- atypical
infiltrates with increased CD4:CD8 ratio with one case of CD8+
MF. Including the case above, there were 28 cases of MF/CTCL with
staging including: 6 stage I, 4 stage II, 3 stage III, 4 stage IV, 3 Sezary
syndrome, 6 large cell lymphoma (LCL) transformation, 2 PTCL and
1 with coexisting Hodgkin’s lymphoma. TCR was positive in 6/14 and
not reported in 14 cases.
Discussion
In 2017, Dupilumab became FDA approved to treat adults
with moderate/severe AD [25]. Dupilumab is a monoclonal IgG4
antibody that blocks the IL4 receptor alpha chain in the shared IL-4/
IL-13 receptor, leading to a decrease in TH2 cell cytokine-mediated
signal transduction [26-28]. Common side effects include injection
site reactions, headache, myalgia and blepharoconjunctivitis [28,29].
In this review, we have summarized a total of 28 cases (including
1 new case from the senior author’s practice) that have a temporal
association with the development of MF/CTCL, a mean time of
18.5 weeks on dupilumab therapy. Park et al and Schaefer et al have
recently reviewed MF/CTCL in association with dupilumab or other
biologic therapies [30,31], but these series did not apply stringent
criteria of at least 6 weeks of dupilumab exposure as we have to
underscore meaningful exposure to this agent in association with the
evolution of MF/CTCL.
Heymann’s commentary highlighted new reports of dupilumab
in prurigo nodularis and bullous pemphigoid that appeared in the
same journal issue as that of Espinosa et al [32], who reported 3
cases of AD developing MF while on dupilumab as well as 3 cases of
MF who received it as adjuvant therapy for itching [12]. No adverse
events were reported in the studies involving PN or BP, although the
patients with AD who eventually were diagnosed with CTCL had
worsening symptoms after various initial periods of improvement.
In the same issue, the editor summarized several cases of MF after
dupilumab treatment and commented on the role of IL-13 excess in
lymphomas and the complex interplay of TH1/TH2 cell responses in
the microenvironments of these diseases by paraphrasing the adage
‘if it’s dry, wet it and if it’s wet, dry it’ as not being the same as ‘if it is
upregulated, downregulate it’ [33]
MF is a malignancy of TH2 T cells, and it is unclear how inhibition
of the IL4/IL13 receptor might facilitate the proliferation of these cells.
It is possible that blocking IL-4/IL-13 signaling on normal/reactive
lymphocytes allows pre-existing small numbers of transformed T cells
to proliferate. This is analogous to prior reports of progressive MF/
CTCL in response to other immunosuppressive regimens, including
classical chemotherapy and calcineurin inhibitors [34]
Table 1a: Mycosis Fungoides presenting after dupilumab therapy- Patient demographics and initial treatment
Abbreviations: MF- mycosis fungoides, CTCL- cutaneous T cell lymphoma, UE- upper extremities, LE- lower extremities, TS- topical steroids, SS- systemic steroids,, TT- topical tacrolimus, NBUVB- narrow band ultraviolet B, PUVA- psoralen plus ultraviolet A, TCR- T cell receptor gene rearrangement, AZA- azathioprine, MTXmethotrexate,
ALK- anaplastic lymphoma kinase, TIA-1- T cell intracellular antigen 1, PTCL- peripheral T cell lymphoma, MMF- mycophenolate mofetil, BSA- body surface area, NR- not reported. *= African american, **Asian
Table 1b: Mycosis Fungoides presenting after dupilumab therapy- Mycosis fungoides presentation and treatment
Abbreviations: MF- mycosis fungoides, CTCL- cutaneous T cell lymphoma, UE- upper extremities, LE- lower extremities, TS- topical steroids, SS- systemic steroids,, TT- topical tacrolimus, NBUVB- narrow band ultraviolet B, PUVA- psoralen plus ultraviolet A, TCR- T cell receptor gene rearrangement, AZA- azathioprine, MTXmethotrexate, ALK- anaplastic lymphoma kinase, TIA-1- T cell intracellular antigen 1, PTCL- peripheral T cell lymphoma, MMF- mycophenolate mofetil, BSA- body surface area, NR- not reported. *= African american, **Asian
It is not possible to tell if any/all these patients had preexisting
MF/CTCL or whether this was de novo transformation into a
malignancy. Due to the relatively short time frame for MF diagnosis,
we review here (median time after dupilumab therapy was 8 months)
and the overall rarity of this presentation compared to the extensive
use of dupilumab in AD, we suspect that atypical T cells were
already existing in many of these patients’ skin prior to dupilumab
therapy. Proliferative T cell neoplasia may have been thus allowed
to progress, much the same as how the gradual diminution of host
immunity with the advancing stage of CTCL has been classically
described to promote progressive disease over time [35]. Additional
hypotheses include the upregulation of IL-13Rα2, a decoy receptor
involved in atopic dermatitis but not blocked by dupilumab [36].
IL-13Rα2 binding by the excess IL-13 could have autocrine effects in
the microenvironment that may further stimulate the growth of the
malignant cells as proposed by Wadele et al [37] and Geskin et al [38]
and previously proposed in the figure published by Hollis et al [13].
Another possible mechanism is that malignant T cells may no longer
bind dupilumab with the same efficacy of normal T cells [37-39].
Conclusion
All these mechanisms remain unproven but have a permissive
effect of dupilumab in mycosis fungoides evolution. Dupilumab has
been a very effective new agent in our armamentarium and these
cases are best seen as a cautionary tale that clinicians pay attention
to the patient’s history and presenting symptoms when initiating this
therapy and have a low threshold for biopsy of any lesions that are not
classic. Atypical/poor responders should be closely followed up and
repeat biopsies considered detecting any unmasked cases of CTCL as
early in therapy as possible.