Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Evidence for Microvesicle Particles in UVB-Mediated IL-8 Generation in Keratinocytes
Bhadri S1, Thapa P1,Chen Y1, Rapp CM1 and Travers JB1-3*
1Departments of Pharmacology and Toxicology, Boonshoft School of
Medicine at Wright State University, USA
2Department of Dermatology, Boonshoft School of Medicine at Wright
State University, USA
3Department of Dermatology, The Dayton V.A. Medical Center, USA
*Address for Correspondence: Travers JB, Department of Pharmacology and Toxicology,
Boonshoft School of Medicine at Wright State University 3640 Col
Glenn Hwy Dayton, OH 45435, USA, Email: Jeffrey.travers@wright.
edu
Submission: 30 November, 2021;
Accepted: 26 December, 2021;
Published: 30 December, 2021
Copyright: © 2021 Bhadri S, et al. This is an open access article
distributed under the Creative Commons Attri-bution License,
which permits unrestricted use, distribution, and reproduction in
any medium, provided the original work is properly cited.
Abstract
Recent studies have implicated bioactive microvesicle particles
(MVP) in the keratinocyte response to many environmental stressors,
in partricular ultraviolet B radiation (UVB). The generation of MVP in
response to UVB involves the Platelet-activating factor receptor (PAFR)
and the enzyme acid sphingomyelinase (aSMase). As UVB generates
some cytokines such as interleukin-8 (IL-8) in a PAFR-dependent manner,
one question is if the production and release of IL-8 and MVP could
be linked. Using the human keratinocyte-derived cell line HaCaT,
the present in vitro studies indicate that pretreatment of HaCaT
keratinocytes with PAFR agonist can synergize with low fluences of UVB
to generate high levels of MVP as well as IL-8 protein. Treatment of
cells with an aSMase pharmacologic inhibitor blocked both processes.
These studies indicate the possibility that MVP could be involved in
pathologic processes involving UVB-generated production of proinflammatory
cytokines such as IL-8.
Introduction
major question in photobiology which has yet to be sufficiently
addressed is exactly how UVB, which only is absorbed appreciably
by the superficial skin epidermis, can generate systemic reactions
[1]. Subcellular particles released by the keratinocyte in response to
environmental stressors have been implicated in this process [2,3].
Specifically, microvesicle particles (100-1000nm, MVP) which are
liberated from plasma membranes, have been demonstrated to be
produced from keratinocytes and human/murine skin in response to
multiple bioactive effector agents including UVB and thermal burn
injury [4-7]. MVP release in response to many of these effectors is
via activation of the Platelet-activating factor receptor (PAFR), with
the enzyme acid sphingomyelinase (aSMase) serving an essential role
in this process [7]. The MVP released from keratinocytes can also
carry multiple protein cytokines and the lipid mediator PAF [6,7].
Regarding the ability of MVP to carry large levels of the metabolically
labile PAF, recent studies using pharmacologic/genetic blocking of
the PAFR and enzyme aSMase have implicated MVP in the systemic
immunosuppressive effects of UVB [7]. The glycerophosphocholinederived
mediator PAF is the most potent lipid-derived mediator
as yet reported [2,3]. Regarding skin and PAF, keratinocytes both
synthesize PAF and related sn-2 oxidized glycerophosphocholines
(Ox-GPCs) and express functional PAFRs linked to generation of
multiple protein and lipid mediators, to include PAF itself [8-10].
UVB irradiation generates both enzymatically generated PAF as
well as non-enzymatically produced Ox-GPC PAFR agonists in
keratinocytes [11], and multiple lines of evidence implicate PAFR
activation in UVB effects. First, UVB-mediated acute inflammatory
responses including pain is decreased in mice lacking PAFRs [12].
Second, studies using cells and mice sufficient/deficient in PAFRs
have demonstrated that expression of many acute genes turned on
by UVB are at least in part PAFR-dependent [10]. It appears that the
PAF system is involved in high fluences of UVB, or in photosensitivity
such as that due to loss of xeroderma pigmentosum complementary
group A [13]. Finally, UVB-induced systemic immunosuppression is
due to the PAFR, in particular the PAFR expressed on the skin mast
cell [14-17]. In contrast, the PAFR does not appear to be involved in
UVB-mediated local immunosuppression [18].
One important aspect of photobiology is the ability of the
environmental pro-oxidative stressor UVB to augment the
pathologic effects of other agents. In vitro studies using keratinocytes
or keratinocyte-derived cell lines have reported that a numbers of
biologic agents such as interleukin-1 β (IL-1β), the phorbol ester
12-O-tetradecanoylphorbol-13-acetate (TPA) and the PAF agonist
carbamoyl PAF (CPAF) can synergize with UVB to generate large
amounts of the potent cytokine tumor necrosis factor-α (TNF-α)
[19,20]. Similarly, our group has demonstrated that CPAF can
synergize with UVB in generating TNF-α in murine skin in vivo [20].
Yet the ability of UVB to synergize with pro-inflammatory stimuli to
augment the production of other cytokines has not been explored.
UVB fluences necessary to generate MVP in keratinocytes, human
skin explants, mice and human subjects appear to be excessive, at
least 2-3 times the minimal erythema dose [5,7]. However, recent
studies by our group have indicated that pretreatment with low-doses
of CPAF or TPA can synergize with UVB in generating MVP in cells,
skin explants and mice in a process involving the PAFR and aSMase
[21]. Yet, the role of MVP in cytokine production is unclear. The
goals of these studies were to test if UVB could synergize with CPAF
to augment the production of the pro-inflammatory cytokine IL-8
and to explore the role of MVPs in this process.
Materials and Methods
Chemicals/UVB:
All chemicals were obtained from Sigma-Aldrich unless
indicated otherwise. UVB of cells used a Philips F20T12/UVB lamp
source (Somerset, NJ, USA) using Kodacel filter to remove UVC (7). The intensity of the UVB source was measured before each experiment using an IL1700 radiometer and a SED240 UVB detector
(International Light, Newburyport, MA, USA) at a distance of 8 cm
from the UVB source.Cell culture:
The HaCaT keratinocyte-derived cell line was provided by Dr.
Petra Boukamp at the German Cancer Research Center, Heidelberg,
Germany [22]. HaCaT keratinocytes were grown in DMEM high
glucose media with 10% FCS as previously described [4,6,7]. HaCaT
keratinocytes were grown to approximately 80-90% confluence in 10
cm dishes, and washed three times with Hanks Balanced Salt Solution
(HBSS) and then incubated with HBSS + 10 mg/ml fatty acid-free
BSA for UVB exposures. Cells were treated with either no treatment,
vehicle (0.1% Ethanol), CPAF, TPA, or the aSMase inhibitor[7,23]
imipramine (50μM) post-UVB radiation. None of the chemicals used
except for imipramine absorbed appreciably in the UVB spectrum.Measurement of MVP and IL-8:
MVP were isolated from cells as previously reported [5-7]. In
brief, cell supernatants were collected and centrifuged at 2,000 x g
for 20 minutes at 4 °C to remove cells and debris. The remaining
supernatant was divided in half for MVP vs IL-8 measurements. MVP
was then pelleted after 20,000 x g centrifugation at 70 minutes at 4°C
from the sample supernatant. The concentrations of the MVP were
determined by using a NanoSight NS300 instrument (NanoSight Ltd,
Malvern Instruments, and Malvern, UK). Three 30-second videos of
each sample were recorded and analyzed with NTA software version
3.0 to determine the concentration and size of measured particles
with corresponding standard error. MVP generated by these model
systems have been previously characterized by western blotting
as expressing Annexin V with only low levels of exosome specific
markers CD63 and Tsg 101, and by transmission electron microscopy
which revealed MVP with appropriate dimensions [7]. IL-8 was
determined via Human Il-8 ELISA kit (R & D Systems) as previously
described [7]. Values were normalized to cell numbers.Statistics
All statistical calculations were performed using Prism 6. Statistical significance determined using two-way ANOVA and the post-hoc Holm-Sidak method, with alpha=5%, or Student t test.
Results and Discussion
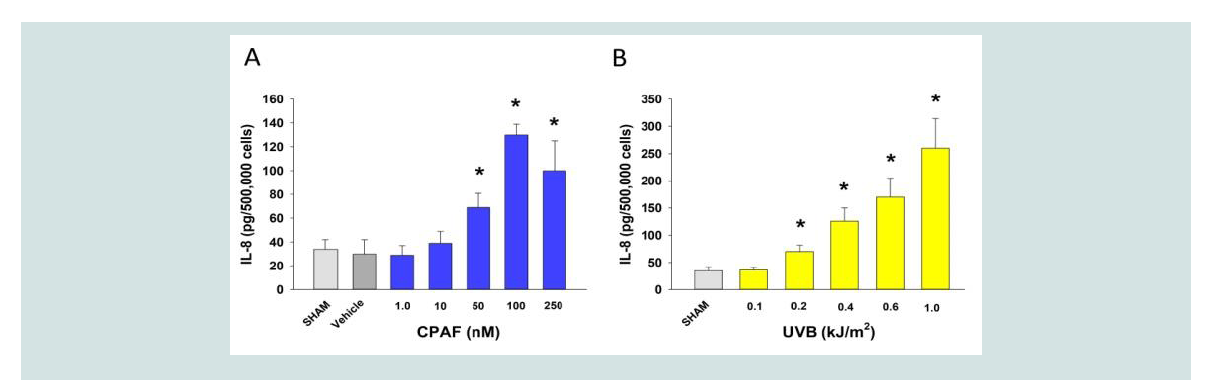
The first studies tested the doseage responsiveness of IL-8 release
in HaCaT epithelioid cells. HaCaT is a spontaneously immortalized
(by heat and high calcium levels) cell line derived from primary
human keratinocytes [22] which expresses functional PAFRs [9]. As
noted in (Figure 1A), treatment of HaCaT cells with the metabolically
stable PAFR agonist carbamoyl PAF (CPAF) resulted in increased
IL-8 release into the supernatants. Exposure of these cell types with
various fluences of UVB resulted in increased levels of IL-8 release at
400 J/m2 and above ((Figure 1B)
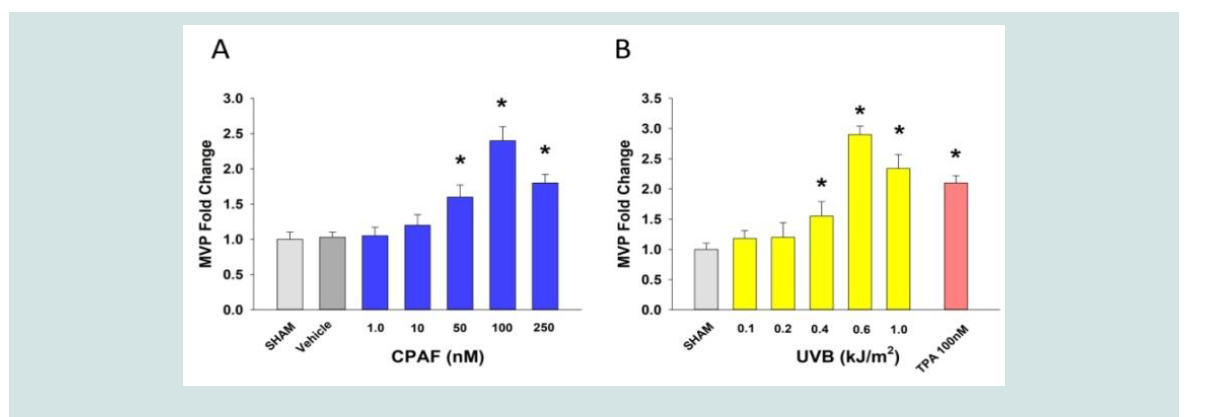
Our next studies examined the dose-responsiveness of MVP
release in HaCaT keratinocytes from the identical supernatants from
(Figure 1). As depicted in (Figure 1), both UVB- and CPAF treatment
resulted in a dose-dependent increase in MVP release into the
supernatants. These findings are similar to those previously reported
by our group [4,7][1]]. Comparison of(Figure 1 and 2) reveal overall
similar patterns of release of IL-8 and MVP into supernatants.
Figure 1: Dosage-responsiveness of IL-8 release by CPAF and UVB in HaCaT keratinocytes. A), B). HaCaT keratinocytes were treated with no treatment (Sham),
0.1% ethanol vehicle, or various concentrations of the PAFR agonist CPAF (A) or fluences of UVB (B) and 4 h later supernatants removed and IL-8 quantified. The
data are mean ± SE IL-8 normalized to cell numbers from three separate experiments using duplicate samples. *Denotes statistically significant (P < 0.05) changes
from sham (UVB) or vehicle (CPAF) by Student t test using Prism 6.
Though UVB has been reported to generate MVP in cells, human
skin explants, mice and human subjects [4-7], the fluences necessary
for MVP release are high. Inasmuch as bioactive mediators such as
PAF agonists, IL-1β, and TPA can augment UVB-induced production
of cytokines such as TNF-α [19,20], we next tested whether these
combinations could result in synergistic release of MVP and IL-8.
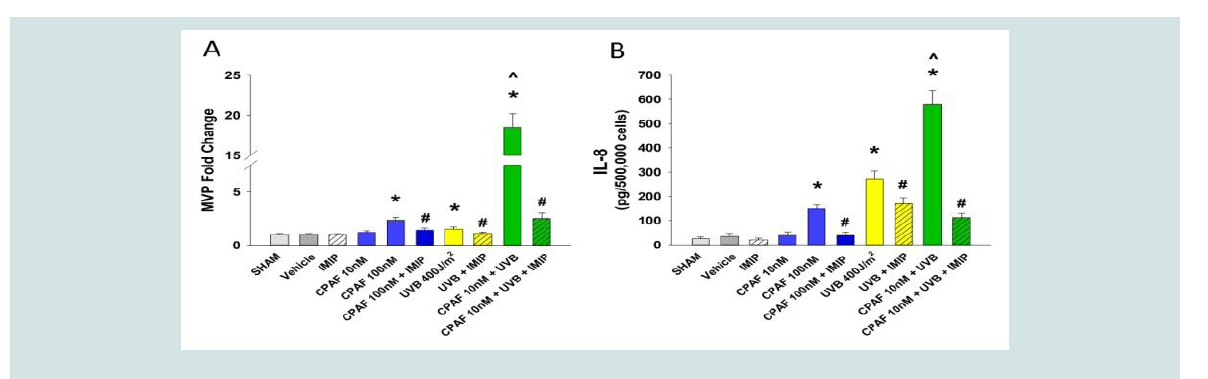
As depicted in Figure 3 pretreatment with CPAF one hour before
fluence of UVB which does not generate significant MVP resulted
in high levels of these subcellular particles in HaCaT keratinocytes.
Of importance, IL-8 levels were also augmented by combination of
CPAF + UVB (Figure 3B).
Figure 2: Dosage-responsiveness of MVP release by CPAF and UVB in HaCaT keratinocytes. A), B). HaCaT keratinocytes were treated as outlined in Figure 1
but MVP quantitated from supernatants. 100 nM of the phorbol ester TPA was used as a positive control. The data are mean ± SE MVP normalized to cell numbers
from three separate experiments using duplicate samples. Baseline levels of MVP were 4.2 x 109 particles per 105 cells. *Denotes statistically significant (P < 0.05)
changes from sham (UVB) or vehicle (CPAF/TPA) by Student t test using Prism 6.
To ascertain a potential linkage between MVP and IL-8 release,
we took advantage of our previous studies indicating that MVP
release in response to CPAF and UVB was dependent upon activation
of the enzyme aSMase [7,23,24]. To that end, we tested the ability of treatment with the aSMase functional inhibitor imipramine following
UVB to block both the MVP as well as IL-8 release. As noted in Figure 3, imipramine blocked the discharge of both MVP as well as the cytokine IL-8.
Figure 3: PAFR agonist augments the release of both MVP and IL-8 in an imipramine-sensitive manner in HaCaT keratinocytes. HaCaT keratinocytes were treated
with no treatment (NT), 0.1% ethanol vehicle (VEH), or various concentrations of the PAFR agonist CPAF, or irradiated with various fluences of UVB. In some
experiments CPAF was given 1 h before UVB irradiation, and 50μM imipramine (IMIP) given immediately after final CPAF/UVB. Supernatants were removed and
MVP/IL-8 quantified. The data are mean ± SE fold change in MVP or concentrations of IL-8 protein normalized to cell numbers from three separate experiments
using duplicate samples. *Denotes statistically significant (P < 0.05) changes by Student t test using Prism 6. #Denotes statistically significant (P < 0.05) differences
induced by imipramine post-treatment compared with CPAF/UVB or the combination using Student t test using Prism 6. ^Denotes statistically significant (P < 0.05)
differences between combination of CPAF + UVB in comparison to either CPAF or UVB alone by two-way ANOVA.
Pathologic responses from multiple agents acting in synergistic
fashion are likely more common than are currently appreciated. An
example of this relating to photobiology is the report of a woman
who developed a severe life-threatening toxic epidermal necrolysis
reaction from the self-treatment of a minor morbilliform drug
eruption from ibuprofen with a single tanning bed exposure [25].
The current studies demonstrate that UVB when combined with a
PAFR resulted in augmented IL-8 release, which adds to previous
studies reporting synergistic TNF-α responses from UVB and other
bioactive agents[19,20]. Consistent with our previously reported
work examining MVP release from keratinocytes and skin [7,21], use of the aSMase inhibitor imipramine indicates this enzyme is involved in both the MVP generation as well as IL-8 release. This finding suggests that MVP and IL-8 release are linked. Moreover, aSMase inhibition could be a tool to dissect the roles of MVP in generation of other cytokines as well as in UVB pathologies.
The role of IL-8 in UVB responses is as yet unclear. However,
the ability of this cytokine to activate and recruit neutrophils suggest
that IL-8 could be important in acute UVB responses. A report using
keratinocyte-fibroblast cocultures that treatment with a neutralizing
antibody against IL-8 blocked UVB-induced production of fibroblast
neprilysin and matrix metalloproteinase I suggest that UVB-induced
IL-8 could have functional consequences [26].
In summary, the current studies reveal that PAFR activation
can synergize with UVB resulting in augmented IL-8 release in a
keratinocyte-derived cell line. The pattern of IL-8 and MVP release
as well as the ability of pharmacologic inhibition of aSMase to block
both processes suggests that IL-8 and MVP are related. Future studies
could define further the roles of MVP and other subcellular particles
such as exosomes in UVB processes including cytokine release.
Acknowledgements
This research was supported in part by grants from the National
Institutes of Health R01 HL062996 (to JBT), R01 ES031087 (to
JBT and YC), and U.S. Veteran’s Administration Merit Awards
5I01BX000853 (to JBT) and 1101CX000809 (to JBT). The contents
of this manuscript do not represent the views of the Department of
Veterans Affairs or the United States Government.