Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Nourkrin® Woman with Marilex® Enhances Hair Growth and Appearance and Improves Hair Confidence in Women with Diffuse Hair Loss from Brazil: An Investigatorinitiated Clinical Study
Mattos Simoes M1, Thom E2* and Wadstein J3
1Av Brasil, 1438, suite 704, Funcionários, Belo Horizonte / Minas Gerais,
Brazil
2ETC Research and Development, Oslo, Norway
3Research and Development, Wadlund A/S, Sweden
*Address for Correspondence: Thom E, Bjornveien 45A, 0774, ETC Research and Development, Oslo, Norway, Tel: 47-91710137; E-mail: Erlingthom@etc.as
Submission: 12 June 2020;
Accepted: 20 July 2020;
Published: 23 July 2020
Copyright: © 2020 Mattos Simoes M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Female Pattern Hair Loss (FPHL) and Telogen Effluvium (TE)
are common dermatological conditions in women, affecting half of the female
population. Treating hair loss in women is more challenging since its pathogenesis
is not fully understood and it commonly leads to more serious psychosocial
consequences compared to hair loss in men. Recent evidence highlights the
involvement of proteoglycan dysmetabolism and follicular hypoglycania as
a mediating pathology. Follicular hypoglycania disturbs cellular activity and
is behind the gradual deterioration of hair follicles, a condition known as
Proteoglycan Follicular Atrophy (PFA). Proteoglycan Replacement Therapy (PRT)
with Nourkrin® with Marilex® is a unique approach that helps to treat PFA and
restore a normal hair growth cycle.
Methods: We aimed to investigate the treatment satisfaction and subjective
efficacy of Nourkrin® with Marilex®. To this end, 67women with moderate to
severe FPHL or TE (mean age=42.73 years) were enrolled into an investigatorinitiated,
subjective, cohort study carried out by practicing dermatologists in Brazil
in collaboration with the World Hair Council. Study participants were randomly
selected by several collaborating dermatologists and have voluntarily started a 6
month course of monotherapy with Nourkrin® Woman (600mg Marilex® per
day). They were interviewed every 3 months using a semi-structured questionnaire.
Results: Just after 3 months, 94.03% and 95.52% of participants reported
having experienced improved hair growth and appearance, respectively; and 83.58%
were more confident with their hair. All Nourkrin® users were satisfied with the
results at this point and were willing to continue with the treatment. At endpoint,
94.03% of subjects experienced enhanced hair growth and 92.54% reported
improved appearance of their hair. These positive changes have led 83.58% of
participants to feel more confident with their hair. Overall treatment satisfaction
rate with Nourkrin® therapy was 97.01%.
Conclusion: Our findings indicate that women with diffuse hair loss found
PRT with Nourkrin® an effective approach that stimulates hair growth and
improves hair appearance. Treated patients felt more self-confident and were
satisfied with Nourkrin® monotherapy.
Keywords
Female pattern hair loss; Proteoglycans; Proteoglycan replacement therapy; Nourkrin®; Marilex®; Patient outcome assessment; Patient satisfaction; Self-confidence
Introduction
Female Pattern Hair Loss (FPHL) and Telogen Effluvium (TE)
are the most common forms of hair growth disorders in women.
Surprisingly, FPHL has just recently been recognised by the scientific community as a separate condition from androgenetic alopecia in
men (also known as Male Pattern Hair Loss (MPHL)). Hair loss is
unexpectedly common among women. Surveys indicate that the
lifetime prevalence of FPHL is more than 55% [1]. This means that
more than half of women would have to withstand the physical and
psychological consequences of hair loss at some point throughout
their life. Although hair loss is not a critical medical condition,
affected individuals often experience significant psycho-emotional
stress that may lead to impaired quality of life and morbid psychiatric
disorders, e.g. general anxiety and depression [2]. Hence, clinicians
are responsible for offering effective yet safe and sustainable treatment
options, specifically to female patients who are more susceptible to
psycho-social complications of hair loss.
Detailed pathogeneses of TE, particularly its chronic form, and
FPHL are not yet fully disclosed. Despite its symptomatic overlap with
MPHL, FPHL is believed to be caused by a different set of aetiological
factors that still wait being determined. In any case, androgens do
not appear to be the sole driver of hair loss in FPHL, since it occurs
in individuals with complete androgen insensitivity syndrome
[3]. New insight has originated from the evidence suggesting that
‘proteoglycan dysmetabolism’ is a central aetiology in diffuse hair loss
that connects the effect of external triggers to degenerative follicular
changes [4,5]. In affected hair follicles, the capacity to synthesize
specific proteoglycans is disturbed, and thus, the concentration of
bioactive proteoglycans at anagen is progressively declined. This
pathology is in clinical literature known as ‘Follicular Hypoglycania
(FHG)’ and causes a defective extracellular matrix, unable to support
the normal growth of follicular cells. Initial stages of FHG cause hair
shedding due to shortening of anagen and elongation of telogen
as in TE and the first stages of FPHL. In the long term, untreated
FHG can lead to gross dysfunction and shrinkage of hair follicles,
known as ‘Proteoglycan Follicular Atrophy (PFA)’. PFA explains the
progressive thinning and miniaturisation of scalp hairs observed in
women with FPHL [4].
Recognising the causal roles of proteoglycans in hair loss and thinning has led to the utilisation of proteoglycan-based therapies
as a novel approach to hair loss. The Originator Nourkrin® with
Marilex®(produced by Pharma Medico Aps, Aarhus, Denmark)
uses a specific combination of bioactive proteoglycans with ‘anagen
inducing’ and ‘anagen maintaining’ properties to mitigate FHG
and treat PFA. This unique method is referred to as ‘Proteoglycan
Replacement Therapy (PRT)’ in clinical literature. Numerous clinical
trials and papers have confirmed and demonstrated the clinical
efficacy and safety of PRT with Nourkrin® in patients with diffuse hair
loss [6-9].
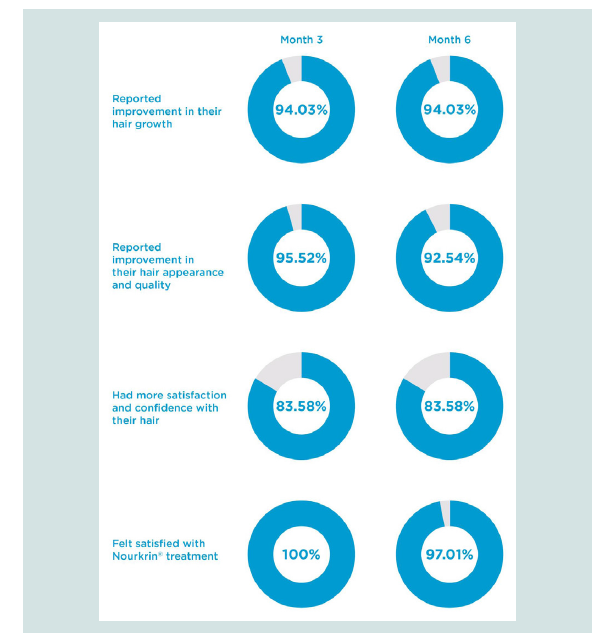
Figure 1: Participants’ impression about various effects of treatment with
Nourkrin® Woman after 3 and 6 months as assessed by a 2-point scale
questionnaire.
In order to provide a more complete picture of the therapeutic
effects, tolerability and treatment satisfaction rate of PRT with
Nourkrin®, a clinical cohort study by practicing dermatologists
was carried out. We have strived in this research to elucidate how
the objective clinical improvements by Nourkrin® are subjectively
perceived by patients.
Materials and Methods
Study participants:
The target population of the current study was female individuals
aged 18 to 65 years with non-inflammatory diffuse hair loss diagnosed
by a qualified clinician. Sampling was performed by independent
collaborating dermatologists in outpatient clinics across major cities
in Brazil, including Sao Paulo, Rio de Janeiro and Curitiba. During the
screening phase, 98 potential subjects were screened from which 74
were enrolled into the cohort phase of the study. Before recruitment,
clinicians gave detailed efficacy and safety information on the
Nourkrin® proteoglycan replacement therapy to eligible patients and obtained their consent for participation. Extra information was also
provided regarding the availability of other therapeutic options.The participants agreed to refrain from taking additional antihair
loss medications or supplements, undergoing laser treatment,
hair transplantation or other major surgical procedures involving the
scalp and maintain their usual hairstyling practices for the duration
of the study. Getting affected by a clinically significant conditionor
taking medications known to affect hair growth (e.g. contraceptive
pills, anabolic steroids, immune modulators and cytotoxic or
cytostatic drugs) during or within six months prior to the study were
considered the criteria for exclusion. Pregnant and breastfeeding
women were also excluded from participation. Subjects with a known
allergy to fish or shellfish were not included as Nourkrin® Woman
contains fish-derived compounds.
Study Design. The present study was an open-label, long-term,
longitudinal cohort with two follow-up evaluations at month 3
and 6. All participants have voluntarily decided to start a 6-month
course of monotherapy with 2 tablets of Nourkrin® Woman (Pharma
Medico Aps, Aarhus, Denmark) per day. Each Nourkrin® Woman
tablet contains 300 mg of an active ingredient, Marilex®. Marilex® is
a proprietary natural extract rich in lecticans and small leucine-rich
proteoglycans with hair growth stimulating properties [4].
At baseline, a collaborating dermatologist interviewed each
potential subject and exerted a general medical and dermatological
evaluation. The severity of hair loss in each patient was graded using
Ludwig classification scale for FPHL [10]. After 3 and 6 months of
treatment, patients were asked to score the changes that occurred in
the growth, quality and appearance of their hair compared to baseline
as well as the effect of the treatment on their hair confidence. For
each evaluation, a structured, self-administered, 2-point (yes or no)
questionnaire was used to assess participants’ self-perception and
overall treatment satisfaction. In addition, questions on the safety and
tolerability of Nourkrin® Woman were included in the questionnaires.
Clinical supervision and scientific consultation were generously
provided by the members of the World Hair Council (WHC)
throughout the study. WHC is a non-profit organization consisting
of trichologists, dermatologists and hair loss specialists dedicated
to improving the lives of people living with hair growth disorders
(https://worldhaircouncil.com).
Results
Out of 74 enrolled participants, 7 failed to report at 3-month time
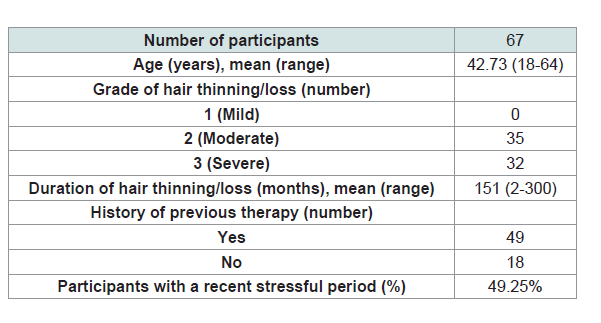
point and thus were eliminated from the final analysis. In this section, data from a per-protocol sample size of 67 is reported. (Table 1) below
presents a summary of baseline characteristics of study subjects. As
shown, participants were middle aged women with either moderate
(52%) or severe (48%) degrees of diffuse hair loss. Despite having a
long history of the condition, more than one third of the patients
have not been diagnosed previously and never been offered an active
medical treatment. Almost half of the subjects reported being under
considerable psychological stress.
Recruited individuals have been asked to judge if Nourkrin®
therapy had a positive effect on their hair and if they feel more
confident with its appearance or not. The obtained results after 3 and
6 months are illustrated in below (Figure 1). Noticeably, the majority
of women with advanced forms of diffuse hair loss have reported
substantial enhancements in the growth and quality of their hair just
after 3 months of Nourkrin® monotherapy. These positive changes
resulted in improvements in hair satisfaction and confidence in 84%
of the cases. Of note, all users (100%) were satisfied with PRT with
Nourkrin® and were willing to continue their treatment after the first
3 months.
At the end of the study period, more than 92% of treated patients
believed that Nourkrin® treatment had significantly improved the
growth and appearance of their hair and 8 out of 10 users expressed
that they feel more confident with their hair than before the
treatment. When asked to score their treatment satisfaction, 9 out of
10 participants expressed their positive overall impression.
Participants were closely monitored during the course of the
study to detect any newly-onset symptoms or side effects. Six
individuals reported minor gastrointestinal, which were of no clinical
significance and did not cause any study withdrawals.
Discussion
The subjects under investigation in this study were a group of
women diagnosed with diffuse scalp hair loss selected by qualified
dermatologists. Reviewing the medical records has brought into light
that around one third of the participants had not received any form
of medical treatment before participating in this study. This troubling
finding implies that many women with hair loss are left undiagnosed
and untreated, which roots in the current poor awareness of Female
Patten Hair Loss (FPHL) and Telogen Effluvium (TE) among both
the public and healthcare professionals. However, even the patients
who are fortunate enough to get noticed and receive medical care are
frequently unsatisfied with the outcomes. Human trials have shown
that more than 50% of women with FPHL do not respond to 2%
topical minoxidil and are unsatisfied with this treatment [11].On the
other hand, finasteride is no more effective than placebo in women
with pattern hair loss [12]. Most patients participating in our study
represent the non-respondent, difficult-to-treat patients that are in
grave need for a novel and effective treatment.
Proteoglycan Replacement Therapy (PRT) with Nourkrin®
is a verified novel therapeutic method that can either be used as a
monotherapy or be added to standard hair loss medications. Hence,
PRT has great potential to occupy an important position in standard
clinical management of hair loss in women, particularly with the
current paucity of effective medications. Marilex® is the active
ingredient in Nourkrin® Woman that comprises of a unique mixture of marine-derived proteoglycans with hair growth stimulating
effects. A recently published, comprehensive review explains how
oral administration of Marilex® can regulate the hair growth cycle and
stimulate dormant hair follicles to start producing new hair [4]. In
two independent randomised, placebo-controlled, clinical trials, 6
months of PRT with Nourkrin® has significantly increased hair density
by 32-36% in patients with pattern hair loss. Subjective assessments
also indicated a high rate of treatment satisfaction in Nourkrin® users
[6,7]. A later trial reported considerable improvements in overall
quality of life and all of its sub-scores after adding Nourkrin® to the
treatment regimen of women with hair loss [9].
Aligned with the abovementioned objective findings, it
was demonstrated in this study that Nourkrin®’s effects are also
subjectively perceived as positive and satisfactory by the patients and
lead to improvements in the self-esteem of affected women. Although
Nourkrin®’s minimum recommended treatment period is 6 months,
continuous use of Nourkrin® Woman for just 3 months produced
noticeable changes in hair growth and quality in more than 90% of the
users (immediate impact). Some experts believe that the immediate
impact of Nourkrin® is fundamentally due to the anagen-inducing
property of the bioactive proteoglycans abundantly found in Marilex®
[13,14]. Improvements in hair growth and appearance persisted
throughout the follow-up period and were judged as significant by
the absolute majority of patients at the end of the study. This was
similar to a previous cohort study conducted in the United Kingdom,
which also revealed comparably high efficacy ratings and treatment
satisfaction with Nourkrin® [8]. One important clinical aspect of
positive patient impression is that not only treatment efficacy results
in higher satisfaction rates, but also patient satisfaction may contribute
to greater symptom resolution and actual disease improvement in a
reciprocal relationship [15].
Of note is that a large proportion of subjects in our study were
under psychological stress at the time of enrolment. It is proven
that stress can independently aggravate hair loss through triggering
an immature anagen termination, promoting telogen, inducing
follicular shrinkage and up-regulating apoptosis [16]. Positive effects
of Nourkrin® on patients’ self-image and self-confidence is hence of
great therapeutic value and can indirectly reduce the severity of hair
shedding. Moreover, in conditions such as hair loss, the primal goal
of treatment is to reduce the psychosocial burden of the disease and
improve patients’ perceived self-image.
In the modern clinical management of hair loss, choosing a
therapeutic approach with an optimal safety/efficacy balance is of
utmost importance. Long-term administration of Nourkrin® Woman
in our study did not induce any side effects. This finding signifies
the safety of Nourkrin® in treating women with diffuse hair loss and
confirms the former observational and interventional clinical reports
[6-9].
Conclusion
Subjective outcomes of long-term PRT with Nourkrin® with
Marilex® were studied in the present longitudinal cohort study.
Participants were women with moderate-to-severe FPHL, selected
from different dermatology clinics in Brazil. Observations were
conclusive as to Nourkrin® therapy produces significant improvements in hair growth and appearance in more than 90% of the patients. 8
out of 10 participants stated that they had more confidence in their
hair after taking Nourkrin® and all volunteers were willing to continue
their PRT after the first 3 months of treatment. Patients’ satisfaction
rate was notably high at more than 97% after both 3 and 6 months of
receiving Nourkrin®. Overall, our findings imply that Nourkrin® is an
effective and safe hair loss treatment that offers tangible benefits to
the patients and equips the clinicians with an extra tool to manage
hair loss in women.
Acknowledgement
Authors would like to thank all the clinicians and assistants
who took part in the enrolment, evaluation and follow-up of the
patients in this study. We also extend our gratitude to Pharma
Medico Aps for supplying Nourkrin® Woman tablets and to the
members of World Hair Council for their tireless contributions.