Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
A Pilot Study on Polidocanol Injection as Treatment for Primary Axillary Hyperhidrosis
Calayan-Terte CMY*, Espinoza-Thaebtharm A and Lopez-Villafuerte L
Department of Dermatology, Jose R. Reyes Memorial Medical Center, Philippines
*Address for Correspondence: Calayan-Terte CMY, Department of Dermatology, Jose R. Reyes Memorial Medical Center, Manila, Philippines, Tel: 63-917 560 8391; E-mail: kimee_calayan@yahoo.com
Submission: 13 February, 2020;
Accepted: 24 March, 2020;
Published: 26 March, 2020
Copyright: © 2020 Calayan-Terte CMY, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Hyperhidrosis is a condition marked by excessive sweating that
can be debilitating leading to emotional and social embarrassment, as well as
occupational, physical and psychological disability. Currently, treatment options
available include pharmacologic and surgical. Pharmacologic treatments include
topical aluminum salts, iontophoresis, systemic medications and botulinum toxin
injection. Meanwhile, surgical treatments include liposuction, direct excision of
the glands, sympathectomy and laser treatment. The aforementioned treatments
provide only temporary results, may have disabling side effects, may be expensive
and some are more invasive that may lead to complications. There are a few studies
regarding the use of sclerotherapy for chemical ablation of the sweat glands to treat
axillary osmidrosis but, none yet for polidocanol and for axillary hyperhidrosis.
Objective: The study aimed to determine the efficacy and safety of polidocanol
injection as treatment for primary axillary hyperhidrosis.
Methods: Patients with primary axillary hyperhidrosis were enrolled.
Identified hyperhidrotic areas were determined and injected with 1% polidocanol.
The degree of hyperhidrosis was assessed using Hyperhidrosis Disease Severity
Scale (HDSS), and Sweating Intensity Visual Scale (SIVS) at baseline, 2 and 4
weeks after injection.
Results: There was percentage reduction in sweating as reflected in the
HDSS and SIVS scores pre-treatment and post treatment. The highest mean
difference was noted between baseline and week 4. Results showed improvement
of hyperhidrosis. However due to the limited number of patients, data was not all
statistically significant. Patients reported slight discomfort after the procedure but
it immediately waned. No other adverse events were noted.
Conclusion: Polidocanol 1% injection may be a promising treatment modality
for primary axillary hyperhidrosis. It is effective and safe, and also inexpensive, less
invasive and with minimal complications compared to ethanol and surgery.
Keywords
Polidocanol; Axillary hyperhidrosis
Introduction
Hyperhidrosis is a condition marked by excessive sweating. It
is a chronic autonomic disorder that can be debilitating leading to
emotional and social embarrassment, as well as occupational, physical
and psychological disability [1]. According to standardized and
validated quality-of-life surveys, the negative effects of hyperhidrosis
are comparable to other conditions, such as severe psoriasis, end-stage
renal disease, rheumatoid arthritis, and multiple sclerosis [2]. In 2004,
Hornberger, et al [3] defined primary focal hyperhidrosis as excessive
[3], bilateral, and relatively symmetric sweating occurring in at least
one of the following sites: the axillae, palms, soles, or craniofacial
region. The following criteria are recommended for diagnosing
primary focal hyperhidrosis: focal, visible, excessive sweating of at
least 6 months duration without apparent cause with at least two of
the following characteristics: a) bilateral and relatively symmetric; b)
impairs daily activities; c) frequency of at least one episode per week; d) age of onset less than 25 years; e) positive family history and f)
cessation of focal sweating during sleep [4]. Secondary hyperhidrosis
on the other hand, can be drug-induced, toxin-induced, caused by a
systemic illness, by congenital disorders or it can be compensatory
[5].
Currently, treatment options available include pharmacologic
and surgical. Pharmacologic treatments include topical aluminum
salts, iontophoresis, systemic medications (i.e. glycopyrrolate,
menthatheline bromide, oxybutynin and clonidine) and botulinum
toxin injection. Meanwhile, surgical treatments include liposuction,
direct excision of the glands, sympathectomy [4] and laser treatment.
The limitations of the aforementioned treatments are: a) provide only
temporary results; b) have disabling side effects; c) may be expensive;
and d) some are more invasive that may lead to complications.
Sclerotherapy is the use of physical, chemical, and biological
properties of an agent used to disrupt target tissue. Sclerosants induce
inflammatory response that result to fibrosis, thrombosis, extraction
of proteins from lipids, denaturation of proteins, cell dehydration
by osmosis, and physical obstruction by polymerization. The result
of these processes is controlled disruption of the targeted tissues’
biologic function [6]. Sclerosing solutions include ethanol, hypertonic
saline, sodium tetradecyl sulfate, polidocanol, sodium morrhuate,
polyiodide iodide and glycerin [6,7]. In dermatology, sclerotherapy is
commonly indicated for the treatment of insufficient veins, recurrent
varicosities and venous malformations [8].
In our literature search, there are only a few studies regarding
the use of sclerotherapy, specifically ethanol, for chemical ablation
of the sweat glands to treat axillary osmidrosis [10,11] but, none yet
for polidocanol and for axillary hyperhidrosis. Hence, this study aims
to investigate the use of polidocanol as a novel therapeutic option in
providing a permanent, cost-effective and less invasive method in
treating primary axillary hyperhidrosis.
Methodology
Study design and study population:
This study is a non-blinded, non-randomized, controlled pilot
study conducted at the Jose R. Reyes Memorial Medical Center
Department of Dermatology. The Institutional Review Board of
this institution approved the study following the guidelines of good
clinical practice.Patients aged 18 to 40 years old, male and female, who tested
positive in the Minor’s iodine starch test and with moderate to severe
hyperhidrosis with a score of 3 or 4 in the Hyperhidrosis Disease
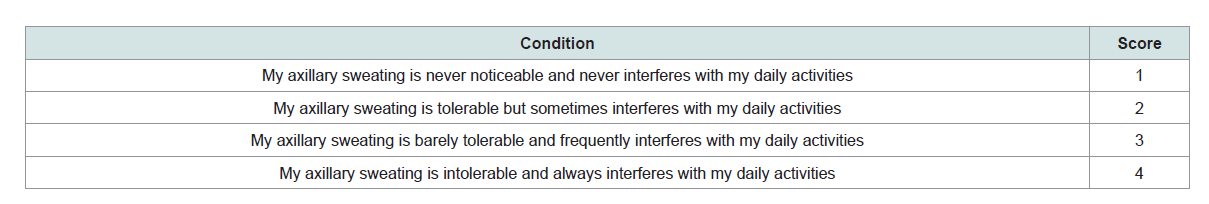
Severity Score (HDSS) (Table 1), were included in the study.
Table 1: Hyperhidrosis Disease Severity Scale (HDSS) [15].
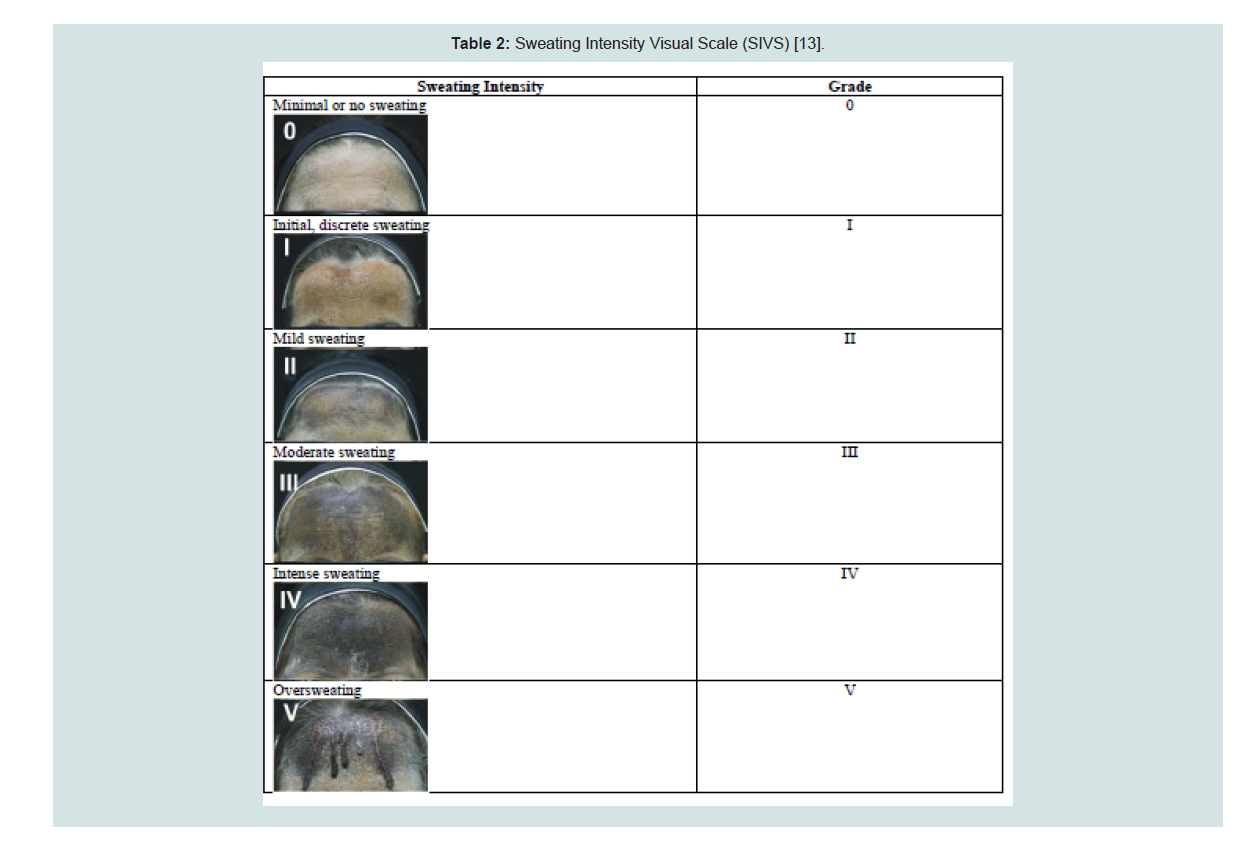
Table 2: Sweating Intensity Visual Scale (SIVS) [13].
Patients who were pregnant or breast-feeding, have secondary
hyperhidrosis, infections or dermatoses over the axillae,
neuromuscular diseases, taking systemic medications that could
interfere with neuromuscular activity and who tested negative in the
Minor’s iodine starch test were excluded. Patients who were using
anti-perspirant were instructed to discontinue its use for a week prior and throughout the duration of the study.
Study intervention and outcome assessment:
The nature and purpose of this study were explained to potential
participants. Six eligible patients were recruited and a comprehensive
written informed consent was obtained. Detailed history was taken
and participants were screened by having them answer the HDSS and
undergoing the Minor’s iodine starch test. The Minor’s iodine starch
test was performed by the primary author by spreading 10% iodine
solution on the axillae, and then corn starch powder was applied after.
After 15 minutes or so, in room temperature of 30-35 °C, the presence
of sweating was indicated by the onset of a dark-blue color [9] on
the participants’ axillae. The Minor’s iodine starch test was evaluated
using the Sweating Intensity Visual Scale (SIVS) (Table 2).The intervention was performed by the primary author under the supervision of the co-authors. Patients were placed in a supine position with their arms abducted to expose the axilla. The identified hyperhidrotic areas from the Minor’s starch iodine test were marked with a dermographic pen and each area was divided into 1 cm2 squares. The treatment areas were cleaned using gauze soaked in sterile water. A dose of 0.1 ml of 1% polidocanol was then injected subdermally into each square using a tuberculin syringe with a gauge 30 needle. A total of 2 ml on the average was injected. Documentation through photography was done at baseline and at specified follow-up periods.
Follow-up periods were at 2 weeks and 4 weeks after 1%
polidocanol injection to assess its efficacy in reducing hyperhidrosis.
The degree of hyperhidrosis were determined by 3 study associates
(primary author’s co-residents) using the SIVS at baseline, 2 weeks
and 4 weeks. Patients answered the HDSS during follow-up visits as
well. Adverse events, such as allergic reaction, pigmentation and skin
necrosis, were also monitored.
Statistical analysis: For the descriptive analysis, means with
their corresponding standard deviations were used to describe the
demographic characteristics of the participants, which include age,
gender and duration of hyperhidrosis. For the HDSS and SIVS pretreatment and post-treatment scores, percentage of reduction was
computed. For the inferential statistics, Kruskal-Wallis is test with its
associated p-value of <0.05 was used to determine if the difference
between the SIVS and HDSS pre-treatment and post-treatment
scores were statistically significant (Figure 1).
Results
A total of 3 patients were enrolled in the study. There were 2
females and 1 male with an average age of 31.67 (age range between
23 to 38 years; SD = ±7.77). The Mean ± SD duration of the axillary
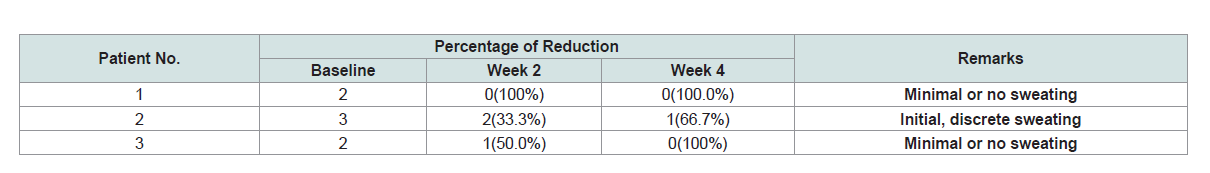
hyperhidrosis was 8.33 ± 1.53 years. Table 3 shows the comparison
of SIVS scores between pre-treatment and post treatment in terms
of percentage of reduction. For patient 1, it was observed with a
100% reduction at week 2 until week 4.Patient 2 also showed 33.3%
reduction at week 2 and remarkable percentage reduction of 66.7% at
week 4. Patient 3 on the other hand had 50% reduction at week 2 and
100% reduction at week 4. The results implied that there was already
a reduction in sweating at a 2-week observation schedule. At the end
of 4 weeks, SIVS scores showed initial, discrete sweating to minimal
or no sweating.
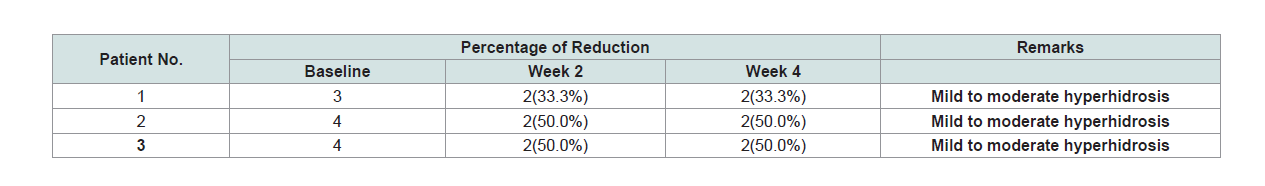
In Table 4, comparison of HDSS scores based on percentage
of reduction is shown. The HDSS baseline scores of patients 1,
2 and 3 were 3, 4 and 4 respectively. The scores of 3 or 4 indicate
severe hyperhidrosis. Patient 1 had 33.3% reduction at week 2 and
continuously at week 4. Moreover, highest reduction percentage was
observed in Patient 2 and 3. Patient 2 and 3 gathered 50% reduction
rate at week 2 and continuously at week 4. The HDSS grading scores
of the three patients is equal to 2 after 4 weeks of treatment. A score of
1or 2 indicates mild or moderate hyperhidrosis.
The patients’ SIVS and HDSS scores were assessed using Kruskal-
Wallis test and the results are summarized in Table 5. The SIVS x2 -
value was 5.213 while the p-value was 0.074. With p-value more than
0.05, there was no significant difference on the SIVS scores between
pre-treatment and post treatment. In contrast, the HDSS x2 – value
and p-value were 7.714 and 0.021, respectively. With p-value less than
0.05, there was a significant difference on HDSS scores between pretreatment
and post treatment.
Results showed that using 1% polidocanol improved primary
axillary hyperhidrosis as reflected in the percentage reduction in SIVS
and HDSS scores, though not statistically significant for SIVS.
Patients reported slight discomfort after the procedure but
it immediately waned. Allergic reaction, pigmentation and skin
necrosis were monitored and recorded. No adverse reactions were
observed in all patients.
Discussion
There are numerous treatment modalities for primary axillary
hyperhidrosis however, not all patients get satisfied with the
results. Removal of the sweat glands offers a permanent solution
to the problem. Various techniques in literature have been used
to achieve this, such as direct surgical excision and liposuction.
Although the success rate of these surgical approaches is high, wound
complications and unsightly scarring are major concerns. In addition,
patients require a long postoperative immobilization and recovery
time [10]. Moreover, the trend in cosmetic medicine is towards
use of noninvasive techniques, but effectiveness and durability are
also important. Patients prefer a shorter recovery time, especially
with cosmetic procedures or treatment of benign conditions [10].
Chemical ablation of the sweat glands using sclerotherapy provides
an alternative approach to surgery. In a study by Hyung-Sup et al
[11] sclerotherapy using absolute ethanol combined with minimal
subdermal shaving was done to treat axillary osmidrosis. Results
showed decrease in malodor and minimal complications, and patients
were satisfied with the outcomes until six months after. Another
study by Han and Li [10] percutaneous ethanol injection for chemical
ablation of sweat glands was used to treat axillary osmidrosis.
Majority of patients (92.1%) considered themselves satisfied with their results. The proposed mechanism of this technique is that when
ethanol is injected into the subcutaneous layer near the interface of
the dermis, where the apocrine and eccrine glands are located, it will
cause necrosis of the glands, while the superficial skin and underlying
vital structures are left intact. However, skin necrosis still developed
in some of the patients [10]. Compared to ethanol, polidocanol has
a better safety profile. It is painless upon injection, does not produce
tissue necrosis if extra vasated, and has a very low incidence of allergic
reactions, although a few cases of anaphylaxis have been reported
[12]. In this study, injection of 1% polidocanol for chemical ablation
of sweat glands, preliminary results showed a significant reduction of
sweating as reflected in both SIVS and HDSS scores, though not yet
statistically significant for SIVS. Moreover, dreaded complications
such as skin necrosis did not develop.
Conclusion and Recommendations
Polidocanol 1% injection may be a promising treatment modality
for primary axillary hyperhidrosis. It is effective and safe, and also
inexpensive, simpler, less invasive and with minimal complications
compared to ethanol and surgery. The authors recommend a larger
sample size because a small number of cases limit statistical analysis.
A longer follow-up period is also recommended to document longterm
effects and for any recurrence. If feasible, a biopsy may be done
to confirm destruction of sweat glands.