Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
In Search of an Innovative Agent for Skin Care - Putting an Ancient Herbal Cosmetic Formula on Modern Bioactivity Testing Platforms
Elaine WAT1,2 Wing Sum SIU1,2 , Helen Yau Tsz CHAN1,2 ,Tiffany Hoi Ka TSO1,2 , Hon Wai LAW1,2, , Ken CHAN1,2, , Chun Wai WONG1,2 , Yan Ping WANG1,2 , Chun Hay KOv,Raymond HU3 , Eric Xing GUO3 , Clara Bik San LAU1,2, , and Ping Chung LEUNG1,2*
1Institute of Chinese Medicine, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong.
2State Key Laboratory for Research on Bioactivities and Clinical Application of Medicinal Plants, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong.
3 5100 Cosmetic Company Limited, 130A, Kwan Tei North Village, Fanling, New Territories, Hong Kong.
*Address for Correspondence
Leung PC, Director, Centre for Clinical Trials on Chinese Medicine(CCTCM), The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, Tel: 852-2252 8868, Fax: 852- 2632 5441; E-mail: pingcleung@cuhk.edu.hk
Submission: 11 April, 2019.
Accepted: 17 May, 2019.
Published: 20 May, 2019.
Copyright: © 2019 Wat E, et al. This is an open access article distributed
under the Creative Commons Attribution License, which permits unrestricted
use, distribution, and reproduction in any medium, provided the original work
is properly cited.
Abstract
1.1.Backgroud: Qi Bai San (QBS) is a traditional Chinese herbal formula
used by ancient ladies for healthy skin and whitening. Nevertheless, it
contains undesirable animal and toxic herbs, without scientific evidence
demonstrating its efficacy.
1.2.Objective: This study aims to compare and identify QBS formula with
the best efficacy from three different versions of QBS formulations, F1, F2,
and F3.
1.3.Methods: Cellular melanogenesis and tyrosinase activity assays were
used to assess melanin content and tyrosinase activity on α-melanocytestimulating
hormone (MSH)- induced B16 cells. Collagenase inhibition
assay was used to compare the collagenase inhibitory activity. Effects
of QBS on melanin production was determined using UV- irradiated
Balb/c mice. Transdermal experiment was used to confirm whether
QBS could penetrate into the skin. in vitro skin toxicity test study was
performed to determine whether QBS would cause toxicity to skin cells.
1.4. Results: F1, F2 and F3 dose-dependently reduced α-MSH-induced
increase in melanin content and tyrosinase activity, and inhibited
collagenase activity. F3 is the simplest formula among all formulations
(without animal or toxic herbs), yet demonstrating similar efficacy. Animal
study suggested F3 could reduce melanocytes and melanin content in UVirradiated
mice. Further penetration and skin toxicity studies suggested
markers from different herbs within F3 could penetrate through the
epidermis to exhibit its effects, without causing toxicity to skin cells.
1.5. Conclusion: We showed for the first time that a modified QBS
formula exert hypopigmentation and collagenase inhibitory effects,
providing in vitro and in vivo scientific evidence supporting its efficacy on
hypopigmentation and healthy skin promotion.
Introduction
Skin, being the most visible organ of the body, serves as the
first and outer most organs to provide barrier against foreign
pathogen [1]. It also serves as a shield against the harmful effects
of ultraviolet radiation emitted by the sun [2]. As we age, we will
experience a decrease in the biological activity, regenerative abilities,
and adaptation of the skin cells. Skin aging can also be affected by
internal factors such as genetics, hormones, nutritional factors,
vitamin deficiencies, as well as external factors such as ultraviolet
radiation, environmental toxins, smoking, improper care, etc. [3].
These external factors, in particularly ultraviolet radiation, aggressive
environments and tobacco smoking can progressively cause damage
to the skin’s cellular and extracellular structures, thereby resulting in wrinkles, sagginess, pigmentation or even neoplastic changes [4].
While these undesirable effects could be minimised with avoidance
of strong sunlight and harmful exposures, topical applications of
various types of agents have been very common practices
The usage of topical agents, also known as cosmetics for the
management of skin conditions and improvement of its outlook is
a common practice among different cultures. The word “cosmetics”
arises from the Greek “kosmeticos” meaning “adorn”. Since then,
any material used for beautification or improvement of appearance is
known as cosmetics [5]. This need for adorning the appearance and
conditions of the face has been an urge in the human race of different
regions and cultures since ancient times, leading to the invention of
different remedies which were handed down for generations with
unabated enthusiasm.
Herbal medicines have been used as skin-whitening agents since
ancient times [6,7]
“Qi Bai San (QBS)” is one of such ancient herbal cosmetic
classic especially used by Royal Family members and dignitaries
as topical agent [7]. According to Yong Lei Qian Fang written by
Zhong Nan Li, a famous Chinese medicine practitioner during the
Yuen Dynasty, QBS was traditionally used for the treatment of skin
pigmentations and consisted of seven different Chinese herbs [8].
This formula may vary slightly but contain herbs of which all in
Chinese language carry the word “White”, signifying its whitening
effects. These herbs include Ampelopsis Radix (dried root tuber of
Ampelopsis japonica (Thunb.) Makino), Atractylodis Macrocephalae
Rhizoma (dried rhizome of Atractylodes macrocephala Koidz.),
Paeoniae Alba Radix (dried processed root of Paeonia lactiflora
Pall.), Bombyx Batryticatus dried body of Bombyx mori Linnaeus, or
the forth to fifth infected instar larvae (or by artificial inoculation)
by Beauveria bassiana (Bals.) to death, Angelicae Dahuricae Radix
(dried root of Angelica dahurica (Fisch. ex Hoffm.) Benth. Et Hook.),
Typhonii Rhizoma, Poria (dried sclerotium of Poria cocos (Schw.)
Wolf), and Tribuli Fructus (dried ripe fruit of Tribulus terrestris
L.) [8]. Recent scientific studies also supported the notion that the
different herbs within QBS are potent on reducing the skin colour
of healthy volunteers receiving recreational exposure to sunlight [9].
Laboratory experiments demonstrated that these herbs from QBS
could significantly inhibit tyrosinase activity, thereby contributing
to their effects in the control of pigmentations [7]. With the several
versions of the formula carrying slightly different components, and
keeping an open mind on the possible compositions, we chose three
formulae of slightly different combinations to be compared in our in
vitro platform studies to identify the formula with the best cosmetic
effects. The most efficacious formula was chosen and tested in our ex
vivo and in vivo platforms to compare its effect with kojic acid alone.
The three formulae were: Formula 1, consisting of Poria; Atractylodis
Macrocephalae Rhizoma; Angelicae Dahuricae Radix; Paeoniae Alba
Radix; Ampelopsis Radix; Bombyx Batryticatus; and Tribuli Fructus;
Formula 2, consisting of the same herbs except Bletillae Rhizoma
was used to replace Angelicae Dahuricae Radix; and Formula 3,
consisting of only 5 herbs of Formula 1 when Tribuli Fructus and
Bombyx Batryticatus were removed.
Overall planning of investigation: To explore the cosmetic effects of the ancient formula which
claimed whitening and smoothening effects, it is envisaged that the
following procedures be taken:
i. Proper authentication of the selected herbs;
ii. Three formulae of slightly different herbal combination will be
prepared to be tested in the same platforms to achieve comparative
effects;
iii. Whitening effects will be studied using melanocyte in vitro
cultures together with collagenase enzyme inhibition tests and
tyrosinase activity assay;
iv. Skin penetration is essential for the topical application of any
topical agent; hence ex vivo tests using porcine skin and diffusion cell
system are to be performed; v. Finally, in vivo experiments using artificially induced skin
melanin pigmentations in C57Bl/6 mice and topical applications of
the formulae will be performed to compare the cosmetic effects;
vi. The final outcomes would reveal a favourable formula and
evidences of its cosmetic effects.
Materials and Methods
Herbal materials authentication and preparation: Herbal material authentication Raw herbal material of Atractylodis
Macrocephalae Rhizoma, Poria, Angelicae Dahuricae Radix, Paeoniae
Alba Radix, Ampelopsis Radix, Bombyx Batryticatus, Tribuli
Fructus, and Bletillae Rhizoma were purchased from a renowned
supplier in Hong Kong. All herbs were chemically authenticated using Thin Layer Chromatography (TLC) in accordance to Chinese
Pharmacopoeia (CP) [10]. Upon chemical authentication, herbarium
voucher specimens of Atractylodis Macrocephalae Rhizoma (2015-
3453), Poria (2015-3454), Angelicae Dahuricae Radix (2015-3457),
Paeoniae.
Alba Radix (2015-3455), Ampelopsis Radix (2015-3456), Bombyx
Batryticatus (2015- 3459), Tribuli Fructus (2015-3458) and Bletillae
Rhizoma (2015-3460) were deposited at the museum of the Institute
of Chinese Medicine at the Chinese University of Hong Kong
(CUHK).
Herbal extract preparation: Extractions of herbs were performed following the traditional
practice of herbal extraction. For each formula, all raw herbs were
mixed in the same ratio and extracted twice by boiling under reflux
at 100 °C using 10x distilled water. The aqueous extracts were then
combined and filtered using cotton wool and concentrated under
reduced pressure at 60 °C. The concentrated extracts were freezedried
and the yield were recorded. All the extracts were stored in
desiccators at room temperature before use.
in vitro cell culture experiments: Cell culture: The B16 melanoma cell line was obtained from
the American Type Culture Collection CRL-6322 (USA). Cells were
grown and maintained in Dulbecco’s Modified Eagle’s Medium
(DMEM) supplemented with 10% (v/v) fetal bovine serum (Gibco,
USA), 100 U/ml penicillin, and 100 μg/ml streptomycin in a 10% CO2
humidified atmosphere at 37 °C. Cells grown to 80% confluence in
T75 culture flasks were trypsinized and seeded into 12- , 24- or 96-
well culture plates for experiments.
Cell viability assay: Briefly, using 96-well plate, B16 cells were
seeded at 4 x 103 cells/well. Cells were then treated with various
concentrations of aqueous herbal extracts in distilled water (0 - 1
mg/ml), with or without 10 nM α-Melanocyte-Stimulating Hormone
(MSH)-induction for 72 hrs. The relative amount of viable cells were
determined by measuring the reduction of MTT dye in live cells to
blue formazan crystals at optical density at 540 nm (Sigma-Aldrich,
USA).
Cellular melanogenesis assay: The amount of melanin present
in α-Melanocyte-Stimulating Hormone (MSH)-induced melanoma
was used as an index for melanogenesis in the present study. B16
cells were seeded onto 24-well plate at the density of 5 x 104 cells/well.
Cells were incubated with 10 nM α-MSH for 72 hrs with or without
different concentrations of aqueous herbal extracts in distilled water
(0 - 1 mg/ml). Cells were washed with Phosphate Buffered Saline
(PBS), followed by trypsinization. Cells were solubilized in 200 μl of
1 N NaOH containing 10% Dimethyl Sulfoxide (DMSO) at 80 oC for
1 hr. The absorbance of all samples were measured at 490 nm [11,12].
2.5 nM kojic acid was used as the positive control.
Cellular tyrosinase activity assay: For cellular tyrosinase
activity measurement, cellular tyrosinase activity was assayed in
terms of DOPA oxidase activity. B16 cells were seeded onto 6-well
plate at the density of 1.2 x 104 cells/well. Cells were incubated with
10 nM α-MSH for 72 hrs with or without different concentrations
of aqueous herbal extracts prepared using distilled water (0 - 1 mg/ml). Cells were sonicated with phosphate buffer (pH 6.8) containing
1 mM Phenylmethanesulfonyl Fluoride (PMSF) (Sigma-Aldrich, St.
Louis, MO. USA.), followed by centrifugation at 10,000 x g. Cell lysate
were mixed with 5 mM L-DOPA (Sigma-Aldrich, St. Louis, MO.
USA.), followed by incubation at 37 oC for 1 hr, and absorbance was
measured spectrophotometrically at 475 nm [13]. 2.5 nM kojic acid
was used as the positive control.
Collagenase activity assay: Collagenase inhibition was
determined using the commercially available Collagenase Activity
Assay Kit (Colorimetric) (Abcam, UK). Test samples were added to
the reaction mixture, followed by addition of 0.35 unit/ml collagenase.
Enzymatic activity was assessed by measuring the reduction at A345
spectrophotometrically for 20 mins. In our assay, 50 μM EGCG was
used as positive control as previously described [14].
UV-irradiation animal study: Animals and diet: All experiments
were carried out in accordance with the guidelines approved by the
Animal Research Ethics Committee at the Chinese University of
Hong Kong (CUHK) (AEEC approval no.: 16/174/MIS). Female
C57Bl/6 mice (8-week old) were supplied by the Laboratory Animal
Services Centre, the Chinese University of Hong Kong. All mice were
housed in normal standard cages (5 animals per cage) at a constant
temperature of 21 oC with a 12-h light-dark cycle. Each standard cage
contained aspen as the bedding material. All animals were allowed
ad libitum access to diet and water. The UVA and UVB spectra used
in this study were produced with a 8W UVB lamp, G8T5E (Sankyo
Denki, Japan) and a 15 W UVA lamp, ST-60 (BOYU Aquarium,
China), and monitored with a UV light meter UV-340A (Lutron
Electronic, Taiwan). To induce pigmentation, female C57Bl/6 mice
were subjected to UV irradiation. Briefly, mice were shaved on their
dorsal trunk 24 h prior to irradiation with 600 μW/cm2 of UVA for 25
minutes each day for 5 consecutive days, then rest for 2 days, followed
by irradiation at 600 μW/cm2 of UVA for 20 minutes, followed by
1200 μW/cm2 (50% UV-A, 50% UV-B) for 5 minutes for another
5 consecutive days. Control mice were shaved and restrained only.
To mimic the clinical situation whereby the topical application is
given as a cream emulsion, herbal extracts (10%) or kojic acid (1%)
were mixed into aqueous cream and applied to the UV-irradiated
mice to serve as test group and positive control group, respectively.
Aqueous cream alone was applied to the UV-irradiated mice to serve
as negative control group. All topical applications were applied to the
mice daily for 1 week. After which, all animals were euthanized by
cervical dislocation, and ear skin was isolated for further analysis.
Melanocyte counting: The melanocyte count in skin tissues was
determined microscopically according to the method of Hiramoto et
al. [15]. The isolated skin tissues were soaked in 2 N NaBr solution
at 37 °C for 2 hrs. Melanocytes were stained by immersing in 0.1
M phosphate- buffered saline (pH 7.2) containing 0.14% L-DOPA
(Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 3 hrs
and the amount of melanocytes were quantified microscopically [16].
Histological analysis of melanin staining: To identify argentaffin
granules and melanin staining within the skin histologically, skins were
fixed in 4% paraformaldehyde overnight at room temperature and
stained for melanin using a Fontana-Masson staining kit (American
Mastertech, Inc. Lodi, CA, USA) according to the manufacturer’s
instructions. Briefly, sliced skins were stained with ammoniacal silver solution for 60 min at 60 °C, followed by incubation in 0.1% gold
chloride and then in 5% sodium thiosulfate. The tissues were stained
with hematoxylin and eosin for morphological analyses.
Localization of tyrosine by immunohistochemistry: Biopsied
skin tissues were embedded in paraffin and cut into 3-mm-thick
sections. To study the cutaneous expression of pigment markers, the
tissue sections were stained and analyzed using a light microscope.
The slides were either stained immunohistochemically stained with
anti-tyrosine antibody (Abcam, UK) for 2 hrs at room temperature.
Specific labelling was detected with a biotin-conjugated anti-rabbit,
anti-goat or anti-mouse IgG and avid in-biotin peroxides complex
(Vector Labs, USA). Immunocytochemistry photographs were
assessed by densitometry [17].
ex vivo diffusion cell experiment: Preparation of porcine ear skin membrane: Porcine ear skin
has been widely used for in vitro transdermal experiments and has
been shown to be the best alternative model for human skin [18]. To
prepare the porcine ear skin for transdermal experiment, briefly, fresh
porcine ear were purchased from a local butcher. Full thickness of the
skin membrane were cut and liberated from the underlying cartilage
using a scalpel, with any adhering subcutaneous fat and tissues
carefully removed. The skin was then cut into 1.77 cm2 sections and
stored at -80 °C prior to use [19].
Diffusion cell experiment: The diffusion experiments were carried out using the diffusion
cell system as previously described[19]. The diffusion cell system
(Taiping Business Mansion, Nanjing, China) comprised of six
diffusion cells, diffusion cell drive, and circulating water bath for
diffusion cells temperature control. Each diffusion cell consisted of a
donor and receiving chamber, with a magnetic stirrer at the bottom
to ensure thorough mixing of the solution at the receiving chamber.
Phosphate buffered saline (Invitrogen, CA, USA) was used as the
diffusion medium at the receiving chamber, with constant stirring at
600 rpm. Temperature was maintained at 37 ± 0.5 °C to ensure all
porcine ear skin membranes’ temperature were kept at approximately
32 °C throughout the experiment. Prior to use, all porcine ear skin
membranes were soaked in pre-warmed PBS for 5 mins to allow
hydration of the membranes before the experiment. All porcine ear
skin membranes were carefully placed on top of each of the diffusion
cells, with dermal side contacting the receiving chambers to avoid the
presence of air bubbles between the membranes and buffer solution.
Donor chambers were placed on top of the membranes. Samples
or PBS (control) were applied to the donor chambers on top of the
porcine ear skin membranes. All diffusion cells were clipped tightly
using cling wrap and horseshoe clamps to avoid evaporation. Receiver
medium was withdrawn after 24 hrs and the sample solution collected
was analyzed using the Liquid Chromatography Mass Spectrometry
(LCMS). Markers within the skin were extracted by sonication in
methanol for 1 hr and subjected to LCMS analysis
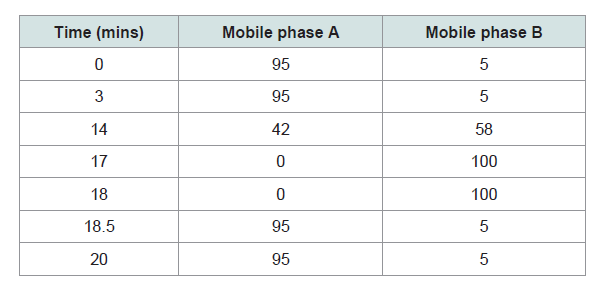
Liquid chromatography mass spectrometry (LCMS)
analysis: LCMS analysis was performed using the Agilent Liquid
chromatography-mass spectrometry LCMS System (Agilent, CA,
USA). Sample solution was injected onto a Waters ACQUITY UPLC
BEH C18 column (100 x 2.1 mm i.d., particle size 1.7 μm) with Agilent Waters ACQUITY UPLC BEH C18 1.7 μm guard column (5 x 2.1
mm i.d., particle size 1.7 μm). All solvents were pre-filtered with 0.45
μm Millipore filter disk (Millipore) and de-gassed. A gradient elution
was carried out using the following solvent systems: mobile phase A
- double distilled water - formic acid (99.9 : 0.1; v/v); mobile phase B -
acetonitrile - formic acid (99.9 : 0.1; v/v). A detailed description of the
gradient elution system used is shown in Table 1. The flow rate used
was 0.5 ml/min. Each sample (5 μl) was injected into the column after
filtration through a 0.2 μm filter disk. Identification of the chemical
markers was carried out by comparing the retention times of the
unknown peaks to those of the standards Table 2. The system was
monitored by a computer equipped with the Agilent MassHunter
Workstation Software for data collection, integration and analysis.
in vitro skin toxicity test: Epiderm culture: A commercially available human epidermal
equivalent, EpiDerm (EPI-200, MatTek Corporation, Ashland, MA,
USA), was used as an in vitro model of the epidermis in this study
since the general morphology of this model system mimicked that
of normal human epidermis. These EpiDerm cultures comprised of
human-derived epidermal keratinocytes, which were cultured on
standing cell culture inserts (Millipore, Billerica, MA, USA) at the
air-liquid interface to form a multilayered, differentiated model of
the human epidermis. Upon kit arrival, the EpiDerm cultures were
placed in 6-well plates, and pre-conditioned overnight at 37 oC and
5% CO2. Samples and positive control (5% SDS) were added to the
skin inserts the next day and allowed to incubate for 1 hr. After which,
all skin inserts were transferred to fresh medium for 24 hrs. All skin
inserts were then transferred to fresh plates for MTT assay.
MTT assay - cell viability test: Skin inserts were transferred to
fresh plates with pre-filled MTT solution, and allowed to incubate for
3 hrs at 37 oC and 5% CO2. Upon completion of incubation, all MTT solution was removed. Skin inserts were transferred to fresh plates
and isopropanol was added to each inserts for 2 hrs for formazan
extraction, before transferring to 96-well plate for spectrophotometric
analysis at 550 nm. Cytotoxicity was expressed as the ratio of the
cell viability, per treatment, to the maximum cell viability from the
negative control (non-treated skin insert).
Statistical analysis: Data were presented as means ± SD for all
in vitro experiments, and means ± SEM for all in vivo experiments.
Prism 5 for Window (version 5.0c, GraphPad Software, Inc., USA)
was used for statistical analysis. Significant differences among all
groups were assessed by one-way ANOVA, followed by Bonferroni’s
Multiple Comparison Test. A probability of p < 0.05 was considered
to be statistically significant.
Results
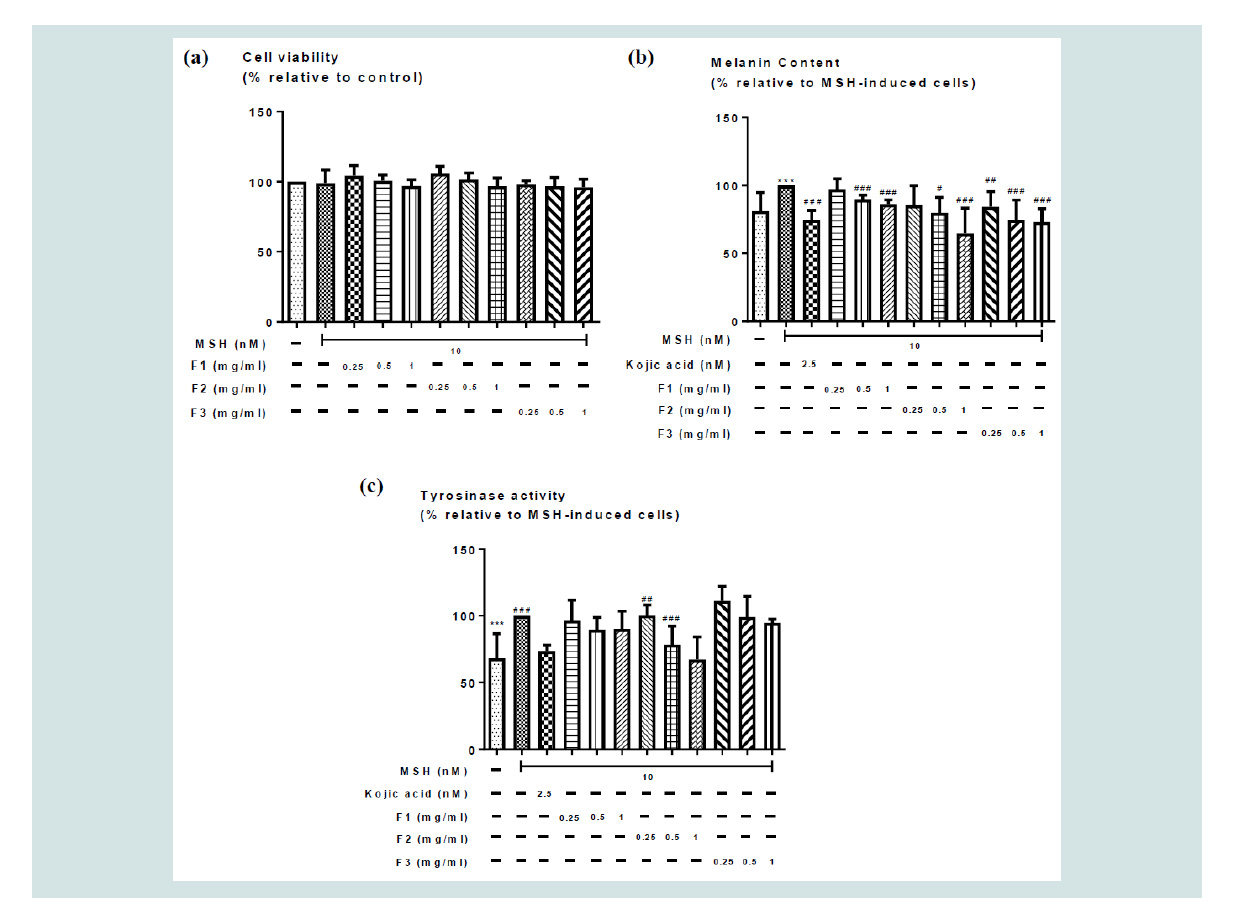
in vitro cell culture experiments: To determine the effects of the herbal extract on hyperpigmentation
in vitro, cell viability assay was firstly used to determine the maximum
non-cytotoxic concentration of the herbal extracts for all 3 herbal
formulae. (Figure 1a) showed the effect of different concentrations of
the aqueous herbal extract with or without MSH induction on the
viability of the cells. MSH at 10 nM had no significant effect on the
viability of B16 cells. There was no significant effect of the aqueous
herbal extracts on the viability of the cells at all tested concentrations
(0 - 1 mg/ml) (Figure 1a), suggesting the aqueous herbal extracts
exerted no cytotoxic effect on B16 cells at all concentrations tested
(0 - 1 mg/ml) and could be used in further in vitro assays.
Cellular melanogenesis assay: The amount of melanin present in α-Melanocyte-Stimulating
Hormone (MSH)-induced B16 cells were used as an index for
melanogenesis in the present study. (Figure 1b) showed the effects
of the different concentrations of the F1, F2 and F3 aqueous herbal
extracts (0 - 1 mg/ml) on MSH-induced melanogenesis. As expected,
10 nM α-MSH induced significant increase on melanin content in
B16 cells. 2.5 nM kojic acid significantly reduced this MSH-induced
increased on melanin content Figure 1b. All F1, F2, and F3 aqueous
herbal extracts dose-dependently reduced the MSH-induced increase
on melanin content. There was also no significant difference on the
melanin content among the kojic acid and aqueous herbal extract
treated groups, suggesting all
3 formulation of the herbal extracts could exert melanin
reduction effect that is comparable to the kojic acid treated group.
There was however no significant difference among all 3 formulae,
although only F3 significantly reduced melanogenesis at the lowest
concentration tested (0.25 mg/ml), while F1 or F2 only significantly
reduced melanogenesis at 0.5 and 1 mg/ml.
Cellular tyrosinase activity assay: For cellular tyrosinase activity measurement, cellular tyrosinase
activity was assayed in terms of DOPA oxidase activity. Figure 1c
showed the effects of different concentrations of F1, F2, F3 aqueous
herbal extracts on MSH-induced increase in tyrosinase activity. As
expected, 10 nM α-MSH induced significant increase on tyrosinase
activity in B16 cells. 2.5 nM kojic acid significantly reduced this MSH- induced increase on melanin content (Figure 1c). Although all
aqueous herbal extracts, could reduce the MSH-induced increase on
tyrosinase activity, only F2 had reached statistical significance. There
was however no significant difference among all formulae.
Figure 1: Effects of the different concentrations of F1, F2, F3 aqueous herbal extracts (0 - 1 mg/ml) on (a) cell viability, (b) MSH-induced melanogenesis; and (c)
tyrosinase activity in B16 cells. Values represent means ± SD (n = 4 - 10). Significant difference between control group and MSH treatment alone group using
Student’s t- test: *** p < 0.001. Significant difference among MSH treated groups with or without co-treatment of different formulae extracts (F1, F2 or F3) using
one-way ANOVA: # p< 0.05, ## p < 0.01, ### p < 0.001. No significant difference among all 3 formulae.
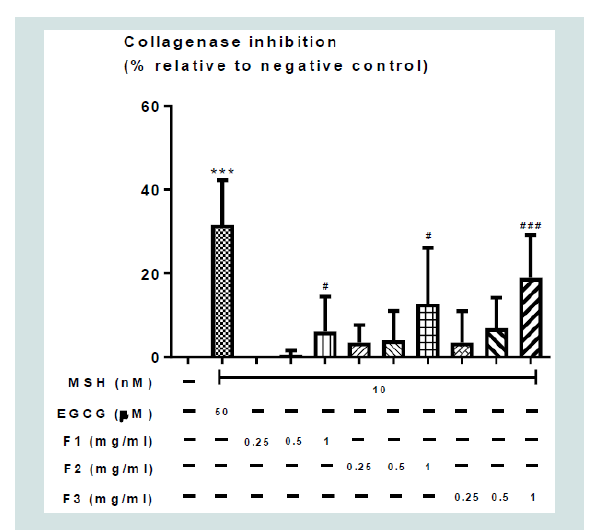
Collagenase activity assay: Figure 2 showed the effects of the different concentrations of F1,
F2, and F3 aqueous herbal extracts (0 - 1 mg/ml) on the inhibition
of collagenase enzyme activity, with negative control demonstrating
samples without any enzyme inhibition, and 50 μM EGCG served as
the positive control. 50 μM EGCG significantly inhibited collagenase
enzyme activity by around 30% Figure 2. F1, F2 and F3 aqueous
herbal extracts dose-dependently inhibited collagenase enzyme
activity, of which F3 appeared to exert the highest inhibition among
all formulae (Figure 2). Nonetheless, this difference did not reach
statistical significance.
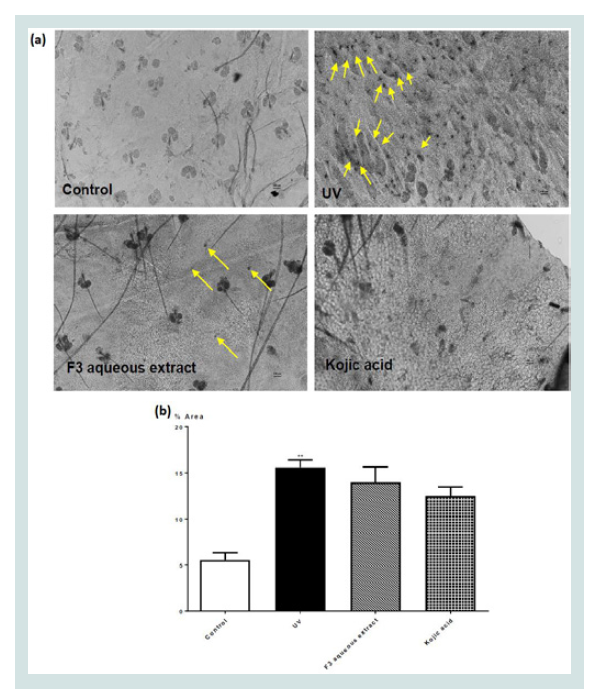
Animal experiment: From all 3 formulae, we had chosen F3 for testing in further animal experiments since F3 appeared to be effective in both reducing
melanogenesis and inhibition of collagenase activity. Figure 3 showed
the effect of different topical treatment on melanocyte production
in the epidermis of ear skin of mice given UV-irradiation. UVirradiation
significantly increased the number of melanocytes in
the epidermis of ear skin in UV-irradiation treated mice Figure 3.
These increase were apparently reduced in the epidermis of ear skin
of mice given different treatment. However, none of the treatment
had reached statistical significance. There was also no significant
difference among all treatment groups. These data are also presented
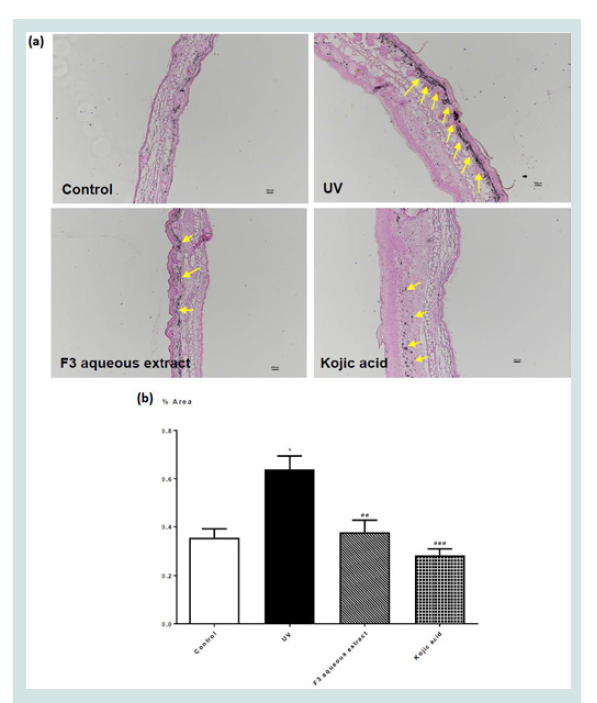
as% area and shown in (Figure 3). Similarly, (Figure 4) showed the effect of different topical treatment
on melanin production in the cross section of ear skin of mice given
UV-irradiation. UV-irradiation had significantly increased melanin
production as evidence by the increased in the% area of skin that were
stained with melanin Figure 4. All treatment significantly reduced the
melanin production. There was however no significant effect among
all treatment groups.
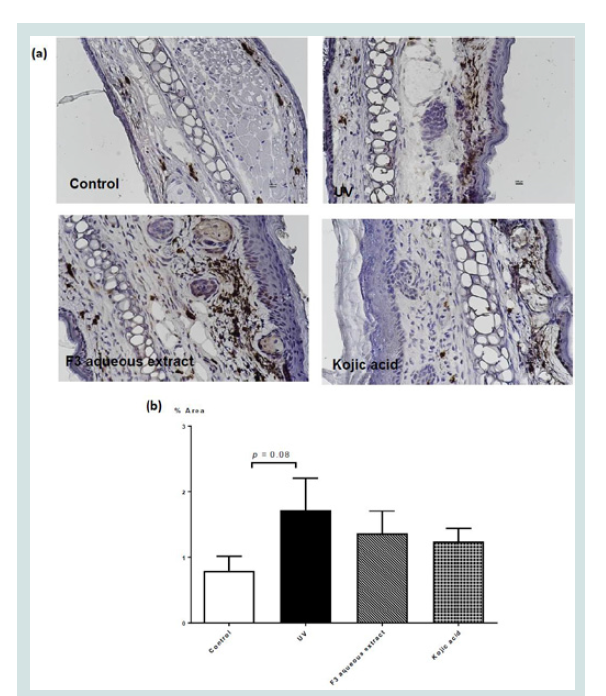
(Figure 5) showed the immunohistochemical staining for localization of tyrosine in ear skin sections of mice given different
topical treatments. Ear skin of mice given UV-irradiation increased%
of area of skin that were stained with tyrosine (p < 0.08). F3 aqueous
extract exerted a trend to reduce this increased in tyrosine localization
which did not reach statistical significance.
Figure 2: Effects of the different concentrations of F1, F2, F3 aqueous
herbal extracts (0 - 1 mg/ml) on collagenase inhibition. Values represent
means ± SD (n = 4 - 8). Significant difference between negative control group
and positive control alone using Student’s t-test: *** p < 0.001. Significant
difference among F1, F2, F3 aqueous herbal extracts (0 - 1 mg/ml) using oneway
ANOVA: # p < 0.05, ## p < 0.01, ### p <0.001. There was no significant
difference among F1, F2, and F3 aqueous herbal.
Figure 3: (a) Dopa staining (x100 magnification) for melanocytes in the
epidermis of ear skin of mice given different topical treatments and (b)
percentage area of the epidermis with Dopa staining in different groups.
Values represent means ± SEM (n = 6 - 8). Significant difference between
control and UV-irradiated control group alone using Student’s t-test: ** p <
0.01. No significant difference was observed among all UV-irradiation treated
groups.
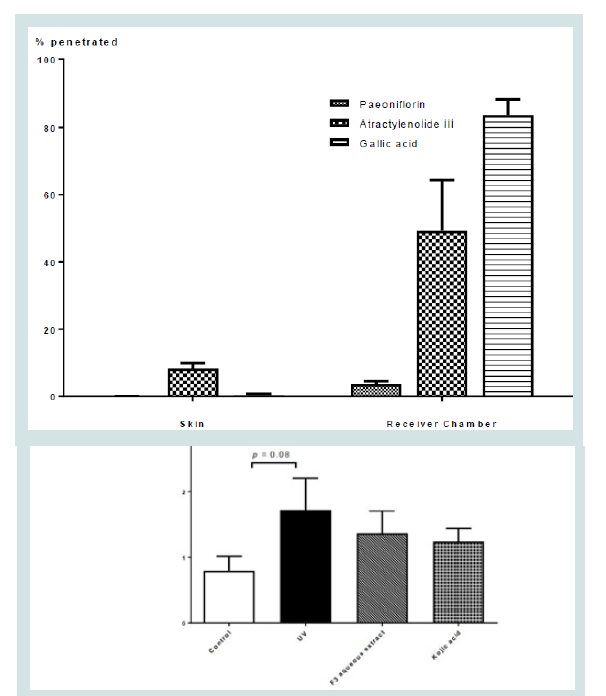
In order for the topical agent to contribute its beneficial effects,
it is important to understand whether the said topical agents could
penetrate through to contribute its beneficial effects using the
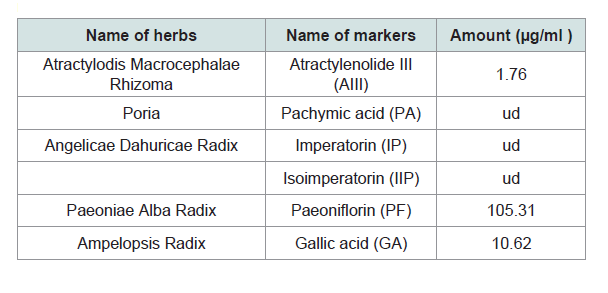
transdermal experiment. Chemical markers for the different herbs
within the aqueous herbal extract F3 was chosen in accordance to CP
recommendations Table 2. Table 2 showed the amount of different
markers contained within the different extract before diffusion.
Markers for Poria (pachymic acid), and Angelicae Dahuricae Radix
(imperatorin and isoimperatorin) were however not detected within
the F3 aqueous extracts. (Figure 6) showed the penetration profile of
the F3 aqueous extract in porcine ear skin after 24 hrs. Upon 24 hours
of penetration, the representative markers from the QBS extract
including atractylenolide III (marker for Atractylodis Macrocephalae
Rhizoma), paeoniflorin (marker for Paeoniae Alba Radix), and gallic
acid (marker for Ampelopsis Radix) were detected within the skin
and receiving chamber after 24 hours of transdermal study, suggested
the formula could penetrate through the skin.
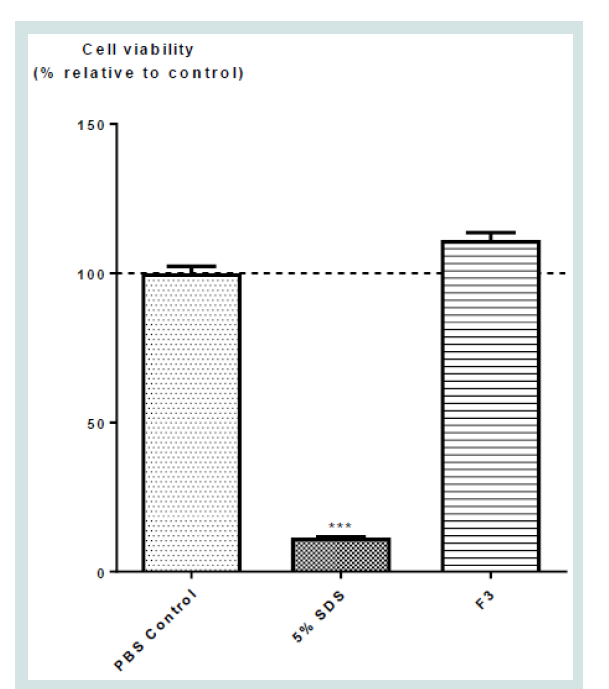
(Figure 7) showed the effects of the different samples on the Reconstructed Human Epidermis using the commercially available
Epiderm in vitro skin toxicity test kit. As expected, positive control
SDS showed the lowest% of cell viability. F3 aqueous herbal extract
had no significant effect on the cell viability, suggesting the formula
exert no toxicity on skin cells.
Figure 4: (a) Fontana-Masson staining (x100 magnification) of melanin in ear
skin sections of mice given different topical treatments; and (b) percentage
area of skin stained with melanin in UV or non-UV irradiated mice given
different treatments. Values represent means ± SEM (n = 4 - 8). Significant
difference between control and UV-irradiated control group alone using
Student’s t-test: *** p < 0.001. Significant difference among all UV-irradiation
treated groups using one-way ANOVA: # p < 0.05, ## p < 0.01, ### p < 0.001.
Discussion
In the present project, we demonstrated the potential beneficial
effect of a simplified and modern form of the classical herbal formula
Qi Bai San, which contains Atractylodis Macrocephalae Rhizoma,
Poria, Angelicae Dahuricae Radix, Paeoniae Alba Radix and
Ampelopsis Radix for promotion of healthy skin. Our in vitro results
suggested that this formula exerted potent effects on the inhibition
of melanin production. This was further supported by our in vivo
results which demonstrated that the herbal formula extract could
reduce UV-induced increase in melanin production and trended to
reduce dopa production and tyrosinase activity. Taken together, these
data suggested the potential of this novel modified form of QBS to be
developed as a topical product for the control of hyperpigmentation.
In the study, we had compared the effects of three different
versions of QBS formulations. We observed significant effect of all
three herbal formula extract on melanin production and tyrosinase
activity inhibition in vitro, with no significant difference observed
among all three formulae. Nonetheless, both F1 and F2 contained Bombyx Batryticatus which is an undesirable animal herb. Bombyx Batryticatus is the dried larva of Bombyx mori L. (silkworm of 4-5
instars) infected by Beauveria bassiana (Bals.) Vuill. It could often
be contaminated with aflatoxin, during the process of storage and
transportation [20]. Aflatoxin is a well-known human carcinogen
[21,22].
Figure 5: (a) Immunohistochemical staining (x400 magnification) for
tyrosinase in ear skin sections of mice given different topical treatments
and (b) percentage area of skin section with tyrosine immunohistochemical
staining in different groups. Values represent means ± SEM (n = 4 - 8).
Significant difference between control and UV-irradiated control group alone
using Student’s t-test: ** p < 0.01. No significant difference was observed
among all UV-irradiation treated groups.
As part of the authentication and standardization process reported
in the Chinese Pharmacopoeia 2015, the quantity of aflatoxin within Bombyx Batryticatus needs to be closely and routinely monitored
[10]. There had also been numerous case reports from different
researchers reporting the poisoning incidents due to Bombyx
Batryticatus [23-25]. Therefore, the use of Bombyx Batryticatus
remains controversial. In seeing so, we had therefore chosen F3, the
only formula without Bombyx Batryticatus for further more thorough
in vivo and ex vivo studies. From our in vivo results, there was a trend
for an improvement on tyrosinase localization. When comparing the
effects between the positive control kojic acid and our herbal formula
extracts, we demonstrated that our modified Qi Bai San F3 exerted
beneficial effects on hyperpigmentation which appeared comparable
or even carried a better trend in potency than kojic acid. Although
kojic acid has been proven to be a potent hypopigmentation agent
and is used widely by various cosmetic company as agents for the
control of pigmentation in the market, increasing concerns are arising
since some animal data suggest kojic acid is weakly carcinogenic [26].
Furthermore, the European Commission’s Scientific Committee
on Consumer Products (SCCP) had also determined that based on
a margin of safety calculation, the use of kojic acid at 1.0% in skin
care formulations poses a risk to human health due to potential
systemic effects. Our modified Qi Bai San, F3 possessing whitening
effects comparable to kojic acid and yet not containing kojic acid, is
therefore of great advantage
Figure 6: Diffusion performance of F3 Herbal formula extracts (% penetrated)
in skin and receiving chamber after 24 hrs.
Figure 7: Effects of F3 Herbal formula extracts on skin toxicity. Values
represent mean ± SD (n = 4). Significant difference between control and all
treatment groups using one-way ANOVA: *** p < 0.001.
Topical cosmetic agent demand genuine improvement on skin
texture, clearance of pigmentations and maintenance of shininess.
Hydroquinone has been used for decades as a hypopigmentation
agent. However, since 2001, its use has been banned for cosmetic
purposes due to the increasing cases of leukoderma-en-confetti/
occupational vitiligo and exogenous ochronosis due to the prolonged
use of hydroquinone. Hydroquinone could also possibly lead to DNA
damage and mutations, causing carcinogenesis [27]. An appropriate
choice could be one that satisfies the demands while maintaining
the natural beauty of the facial cover. An ancient cosmetic formula
inherited from the Imperial Court of the six century Tang Dynasty
is the target of the present study. While the related stories appear
convincing, the composition of most herbal natural products is wellknown
for slow, mild activities. What need to be provided before its
proper endorsement would be evidences of its skin protection and
safety.
We applied in vitro and in vivo experiments to demonstrate the
de-pigmentation bioactivities of the formula as well as its protection
effects of skin texture. When comparing three slightly different
combinations of herbs, we prefer the simplest one which excludes
animal and toxic items. The herbs chosen are also contained in the
Inventory of Existing Cosmetic Ingredients (IECIC) which will
facilitate the future commercialization of the finished product. With
the intriguing in vitro and in vivo results, it would be reasonable to
move on to the next step to conduct a pilot clinical study comparing
the effects of the herbal formula with the commonly used treatment for
hypopigmentation clinically such as the kojic acid and hydroquinone
groups.
In conclusion, our present results have provided pre-clinical
scientific support from in vitro to in vivo studies for the efficacy of a
modified form of the traditional, and yet well-known herbal formula
Qi Bai San on UV-induced hyperpigmentation. The beneficial effects
of our modified Qi Bai San is comparable to the commercially positive control of kojic acid. These data provided support for this novel
herbal formula to be developed as a cosmetic product for marketing
in the near future.