Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Safety and Efficacy of Eczero®, (Ochrocarpus longifolius)Cream in the Treatment of Mild to Moderate Eczema - An Open Label, Phase II, Non-Comparative Study
Kavita Salkar1*, Chetna Chotalias1, Nimesh Mehta2 and Anirudh Tripathi3
- 1Phytomedicines Department, Piramal Enterprises Limited, India
- 2Clinical investigator, Sai Medi Centre, India
- 3Clinical investigator, Life Veda Treatment and Research Centre, India
*Address for Correspondence: Kavita Salkar, Phytomedicines Department, Piramal Enterprises Limited, Mumbai, India, E-mail: pincelli.thais@mayo.edu
Citation: Salkar K, Chotalia C, Salvi R, Mehta N, Tripathi A. Safety and Efficacy of Eczero® (Ochrocarpus longifolius) Cream in the Treatment of Mild to Moderate Eczema - An Open Label, Phase II, Non-Comparative Study. J Clin Investigat Dermatol. 2018; 6(2): 6
Copyright: © 2018 Salkar K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited..
Journal of Clinical & Investigative Dermatology | ISSN: 2373-1044 | Volume: 6, Issue: 2
Submission: 09 September, 2018| Accepted: 05 October, 2018| Published: 12 October, 2018
Abstract
Background: Eczero® cream is developed from Ochrocarpus longifolius plant extract studied for its anti-bacterial and antiinflammatory properties.
Objective: To evaluate the safety and efficacy of Eczero® cream in treatment of mild to moderate eczema.
Method: This was an open label, non-comparative, phase II, clinical trial conducted in 44 patients. The treatment was given for a period of 28 days. Overall eczema severity was assessed by using local SCORAD index, VAS score for itching and patient and physicians global assessment of therapy.
Results: Application of Eczero® cream showed significant reduction in edema, erythema, lichenification and oozing with an overall improvement in the eczema lesions. The cream was well tolerated with no serious adverse events observed
Conclusion: Eczero® cream can be considered as a possible therapeutic option for the treatment of mild to moderate Eczema in adults.
Keywords
Eczema; Clinical study; Eczero; Ochrocarpus longifolius
Introduction
Eczema is a chronic, relapsing, inflammatory skin condition characterized by an itchy red rash. Eczema affects both sexes equally and usually starts in the first weeks or months of life. Acute eczematous lesions are characterized by erythema, oozing and crusting, whereas chronic lesions show papules and lichenification. Pruritus is so prominent and constant that it has an effect on quality of life. The pathophysiology of Eczema is still unknown and many theories are believed which involves immune dysfunction, allergies, genetic factors and some non-allergic conditions like irritants, contact allergens, stress factors and secondary infection by Staphylococcus aureus [1-3]
The social and economic impact of eczema is considerable, especially when the disease is severe, with sufferers experiencing significant limitations of normal social functions. It has a profound impact on the quality of life of both children and adults [4]. General treatment includes use of emollients and topical steroids along with antibiotics. However this current treatment for eczema has certain limitations. Issues related to long-term safety, adverse events like skin thinning and potential link with cancer are some of the problems associated with the available different treatment options for eczema [5].
The use of flower buds of Ochrocarpus longifolius is mentioned in Indian ancient literature [6,7].
Flowers buds are aromatic, possess carminative and astringent properties and are used for hemorrhoids, dyspepsia, gastritis, leucoderma, headache and snake and scorpionbite in traditional medicine [8].
In our regular screening program we identified, Ochrocarpus longifolius as an active plant against S. aureus and MRSA strains. The plant was studied in various in vitro models for its antibacterial potential. Positive results were obtained in our preclinical studies in which the plant extract showed MIC values of 1 to 4 mcg/ml against various strains of S. aureus including the clinical isolates and antibiotic resistant strains of S. aureus (MRSA) [9]. Further the plant also showed moderate anti-inflammatory activity. These findings induced us to develop a topical cream for eczema conditions. The developed formulation was also tested in in-vitro models for efficacy and toxicity studies and promising results were obtained in these studies.
In view of these conditions our developed formulation of topical cream with active plant extract possessing potent antibacterial and anti-inflammatory activity presents a good option for treatment of eczema in a natural way. The objective of the present study was to determine the effectiveness of Eczero® cream in reducingthe severity and intensity of signs and symptoms of mild to moderate eczema along with assessment of reduction in inflammation and lesion; relief from itching and assessing the safety of cream in patients.
Materials and Methods
Preparation of investigational product
The investigational product was Eczero® cream, a topical formulation for external application. Eczero® cream contains 2% plant extract prepared from flowering buds of Ochrocarpus longifolius (Table 1).
Study population
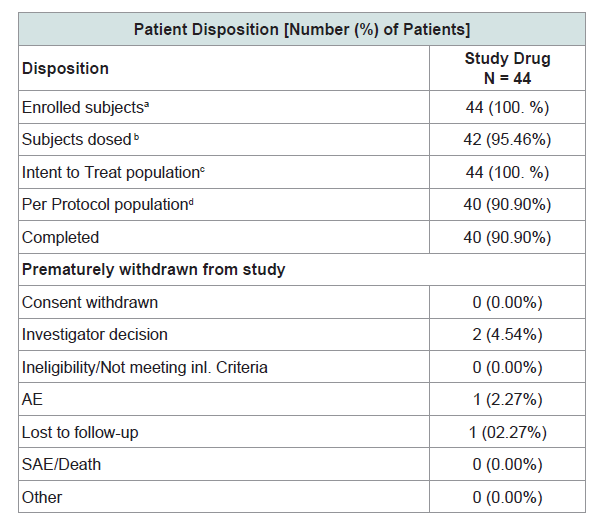
In this study 44 Patients were enrolled from 2 centers in India. The study was conducted in accordance with the ethical principles described in the declaration of Helsinki, and the ethics committees reviewed the protocol and granted approval before the start of the study. A screening visit was carried out on day 1. All patients were provided written informed consent before study participation. Adult male and female subjects of ≥18 years and ≤ 75 years, having chronic eczema (suffering from eczema from the past 1 year) with an EASI (Eczema Area Severity Index) score in the range of 7-15 (both inclusive), not having any systemic immunosuppressive therapy (eg steroids) were included in the study (Table 2).
Study design and disposition
The study was an open label, phase 2, non-comparative study to evaluate the effect of Eczero® cream in patients suffering from mild to moderate eczema. This was a single arm study with all patients receiving the Eczero®cream. The trail is registered with clinical trial registry of India and registration number is CTRI/2018/01/011211 (http://www.ctri.nic.in/).
The study drug was administered after allocation of patients to the two sites in the study and the safety evaluation at the baseline visit. Patients were advised to apply the cream on affected area on entire body. To check the efficacy of the cream in treating eczema, half to one FTU (finger tip unit) was applied three times a day for a period of 28 days and the compliance was recorded in the case report form (CRF).The efficacy of Eczero® cream was evaluated on 7th, 14th, 21st and 28th day. The drug accountability was checked by recording in CRF for remaining or unused quantity of cream in terms of grams of the total unused medication.
Assessment
The efficacy of Eczero® cream was evaluated by studying the reduction in the severity of symptoms of mild to moderate eczema measured by Local SCORAD Index on Screening / Baseline, Day 7, Day 14, Day 21 and Day 28. SCORAD score clinical scoring was based on six parameters viz. erythema, excoriation, edema/population, oozing/crusts, lichenification and local pruritus. Each of these parameters were graded on a 4 point scale (0-3) wherein 0 = absent, 1= mild, 2 = moderate, 3 = severe. After grading the efficacy parameters during each visit the total added scores for all the 4 efficacy parameters provided the “clinical score”. A marked clinical response was considered to be reduction of 3 or more in the clinical score to a final value less than 3 or final score of zero.
Secondary efficacy parameters included measurement of reduction in inflammation, lesion session and itching measured using VAS for pruritus, physicians and patients global assessments. The efficacy parameters and adverse events along with concomitant usage of medicine during the treatment were measured at every follow up visit. Photographs of the target lesion were taken at the baseline and at the end of the treatment. The treatment compliance of the medicine used by the patients was recorded on every follow-up visits. The safety parameters like CBC, SGPT, Sr. Creatinine were measured on the screening day and day 28, while only X-ray was done on the screening day. All the patients were instructed to not to apply or take orally any kind of other medication during and till the end of the study.
Statistical analysis
Data was analyzed using SAS 9.4. Data was represented as Mean ± SD or Frequency (Percentage) as per the type of data. Multivariate ANOVA test (MANOVA), Wilks’ Lambda statistical tests were used to compare the covariance between baseline and end of treatment groups for eczema (Erythema). Similarly Wilks’Lambda, multivariate statistical tests were used to compare the covariance between baseline and end of treatment groups for excoriation, edema/population,oozing/crusts, lichenification and local pruritus. Chi-square test was performed as an appropriate measure of association between two categories for Physicians Global Assessment (PhGA) with photographic assessment and PatientsGlobal Assessment (PtGA).
Results
Patient demographic and other baseline characteristics
In view of these conditions our developed formulation of topical cream with active plant extract possessing potent antibacterial and anti-inflammatory activity presents a good option for treatment of eczema in a natural way. The objective of the present study was to determine the effectiveness of Eczero® cream in reducingthe severity and intensity of signs and symptoms of mild to moderate eczema along with assessment of reduction in inflammation and lesion; relief from itching and assessing the safety of cream in patients.
Primary efficacy analyses
Multivariate ANOVA test (MANOVA), Wilks’ Lambda and Pillai’s trace multivariate statistical tests were used to compare the covariance between two sites (groups) for eczema symptoms viz. Erythema, excoriation, edema/population, oozing/crusts lichenification and local pruritus (Table 3).
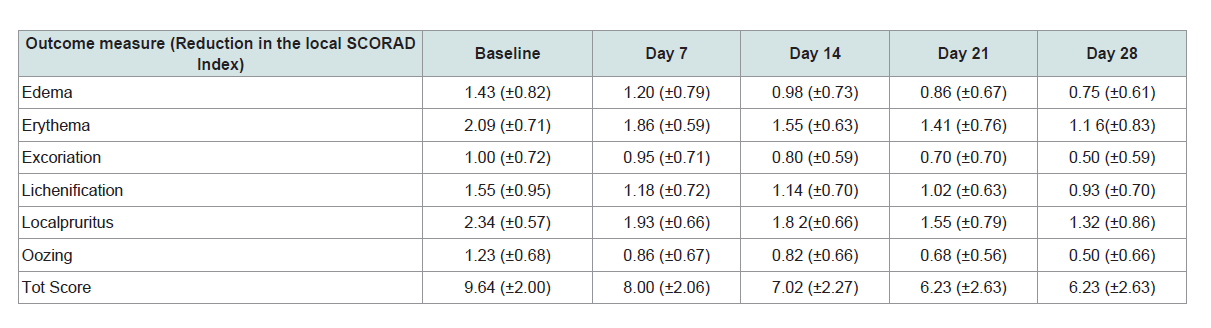
The severity of disease was calculated quantitatively on a Likert scale with the attributes like edema, erythema, excoriation, lichenification, pruritus and oozing. The mean Baseline score for edema, 1.43 ± 0.82 on Likert scale reduced to 0.75 ± 0.61 on Day 28 Visit, for erythema 2.09 ± 0.71 it reduced to 1.16 ± 0.83, lichenification 1.55 ± 0.95 reduced to 0.93 ± 0.70 and oozing 1.23 ± 0.68 reduced to 0.5 ± 0.66. The mean Baseline score for excoriation 1.00 ± 0.72 on Likert scale reduced to 0.50 ±0.59 on Day 28 Visit and local pruritus 2.34 ± 0.57 reduced to 1.32 ± 0.86. The mean Baseline score for total SCORAD score 9.64 ± 2.00 on Likert scale reduced to 6.23 ± 2.63 on Day 28 Visit.
Application of Eczero® cream showed significant reduction in edema [F (5, 38) = 9.68, p = <0.0001], erythema [F (5, 38) = 9.42, p = <0.0001], lichenification [F (5, 38) = 7.93, p =<0.0001], and oozing [F (5, 38) = 4.16, p = 0.0041] whereas the symptoms like excoriation [F (5,38) = 1.98, p = 0.1039] and pruritus [F (5, 38) = 1.76, p = 0.1456] does not showed significant outcome. However, the VAS score assessed to evaluate the pruritus as the immediate complaint of eczema showed significant reduction of pruritus [F (5, 38) = 9.68, p = <0.0001]. The primary efficacy parameter total SCORAD score showed significant reduction as compared to the baseline visit associated F value, and p value [F (5, 38) = 7.12, p = 0.0002].
The Wilks’ value for edema (0.439772), [F (5, 38) = 9.68, p = <0.0001], erythema (0.446527), [F (5, 38) = 9.42, p = <0.0001], lichenification (0.489388), [F (5, 38) = 7.93, p = <0.0001] and oozing (0.646341), [F (5, 38) = 4.16, p = 0.0041] showed significant differences observed between baseline to Day 7, 14, 21 and Day 28. The Wilks’ value for excoriation (0.793365), [F (5, 38) = 1.98, p = 0.1039] and local pruritus (0.812342), [F (5, 38) = 1.76, p = 0.1456] showed no significant differences observed between baseline to, Day 7, 14, 21 and Day 28. The Wilks’ value for total SCORAD (0.571723), [F (5, 38) = 7.12, p = 0.0002], showed significant differences observed between baseline to, Day 7, 14, 21 and Day 28.
Thus various statistical tests used to evaluate the signs and symptoms of eczema for Eczero® cream showed reduction in the eczema symptoms at the end of the study. The total SCORAD score showed a significant reduction at day 28 from baseline in all the groups.
Secondary efficacy assessment
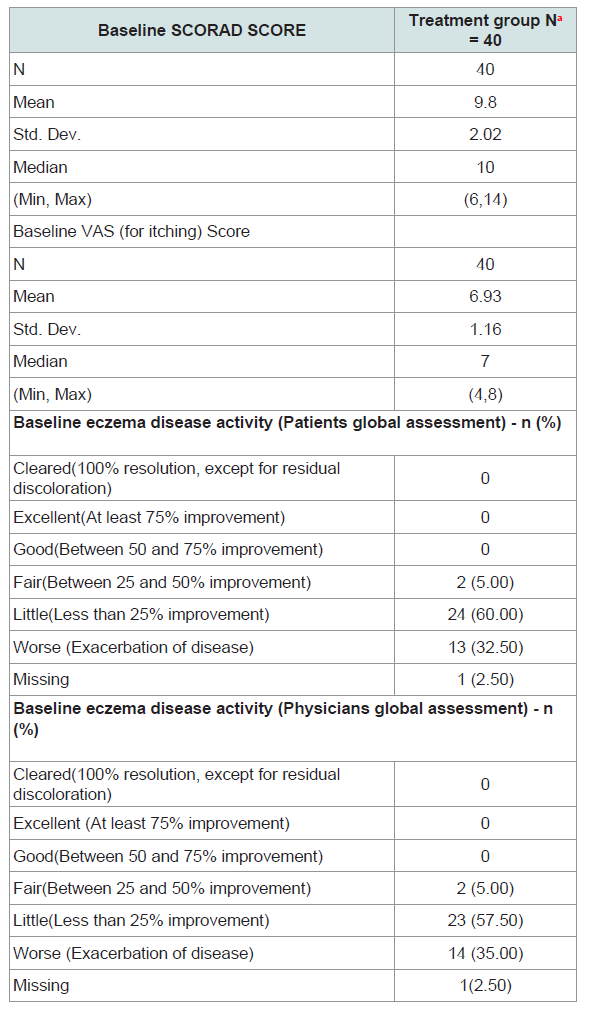
Often, pruritus is the first symptom of eczema relapse. VAS is a 10-cm long line (oriented horizontally or vertically); on which patients indicate the intensity of pruritus by crossing the line at the point that corresponds to their pruritus severity. In our trial the scale indications were as follows: 0 = no pruritus, > 0-< 4 points = mild pruritus, ≥ 4-< 7 points = moderate pruritus, ≥7-< 9 points = severepruritus, and ≥ 9-10 points = very severe pruritus. VAS was evaluated on Day 7, Day 14, Day 21 and Day 28 of the trial. The mean VAS score was recorded to analyse relief from pruritus; mean baseline score of pruritus was 6.93 ± 1.13 SD on scale of 10 cm, reduced to 4.00 ± 2.27 SD on Day 28 Visit. The Wilks › value for this test (0.439772), its associated F value, and p value [F (5, 38) = 9.68, p = <0.0001], showed significant differences observed between baseline to Day 7, 14, 21 and Day 28 (Table 4).
Physicians and patients global assessment
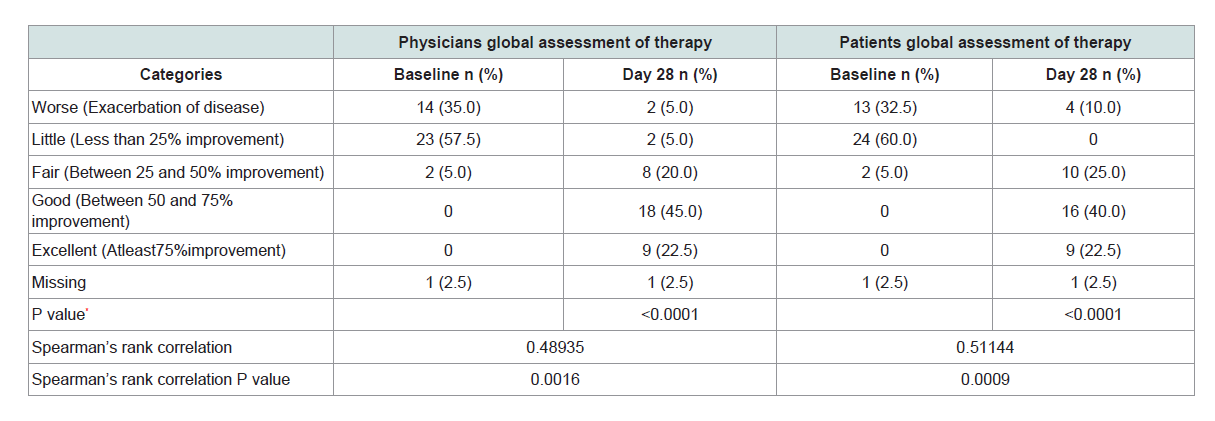
Physician’ global assessment of therapy with photographs of lesion was recorded on Baseline/screening (week 1) and on last followup visit in Week 4 to assess the overall improvement in the lesion. For Physician’ and patients Global Assessment; the severity was assessed on a five point scale, as Worse = 1 (Exacerbation of disease), Little = 2 (Less than 25% improvement), Fair 3 = (Between 25 and 50% improvement), Good = 4 (Between 50 and 75% improvement) and Excellent = 5 (At least 75% improvement) As per physician’s global assessment almost 22.5% (9) patients’ showed excellent results based on photographic assessment, whereas 45% (18) patients received good results. Similar trend was observed for Patients global assessment where 22.5% (9) patients’ showed excellent results, 16 (40%) showed good results and 10 (25%) showed fair results. Mann-Whitney U test was used to compare the scoresof Baseline and Day 28. P value observed was less than 0.05 hence; there was significant difference between baseline and Day 28. Chi-Square test was used to measure association between two categories- Eczema (PhGA) and Eczema (PtGA). P value observed was less than 0.05 which showed a significant association between PhGA and PtGA (Table 5).
Safety and adverse events
Safety assessment during study included monitoring of the adverse events and vital signs as well as the clinical laboratory evaluations. All 40 patients in per protocol population completed the study. No clinically significant changes were observed for the vital signs of temperature, systolic blood pressure, diastolic blood pressure, pulse rate and respiratory rate. A total of 5 adverse events were observed in 4 patients under various systems. Organ Class of Skin and Subcutaneous disorders: One adverse event of Pruritus; Gastrointestinal disorders: two adverse events (Diarrhea 1, Constipation 1) and Respiratory system: 2 adverse events (common cold and cough). The adverse event of Pruritus in Eczero®group may be related to the hypersensitivity, other events like diarrhea, constipation, common cold and cough were unrelated to the treatment product. No severe adverse events (SAEs) were observed with Eczero® treatment in the patients.
Discussion
The present study is a first of its kind study to investigate the clinical efficacy and safety of Ochrocarpus longifolius plant based Eczero® cream in subjects with Mild to Moderate Eczema. Wedesigned a 4 week study to determine the effectiveness of Eczero®cream in reducing the severity and intensity of symptoms in patientssuffering from mild to moderate eczema.
The results indicate that the application of study product thrice a day for 28 days reduced the signs and symptoms of eczema. During the study period, no adverse events were reported, suggesting that daily application of the study product Eczero® cream is safe and welltolerated in patients suffering from mild to moderate eczema.
Staphylococcus aureus a gram positive bacterium which is a normal flora of human skin has a peculiar ability to colonize the skin of patients with eczema and Atopic Dermatitis (AD) and is consistently found in eczematous skin lesions in these patients. The skin lesions of 80-100% of patients with eczema and AD are colonized with S. aureus. In contrast, S. aureus can be isolated from the skin of only 5-30% ofnormal individuals, mainly from intertriginous areas. A correlation between the severity of the eczema and colonization with S. aureus has been demonstrated, and it has been determined that bacterial colonization is an important mechanism aggravating skin lesions. It has been demonstrated in various studies that Staphylococcus aureusinfection is related to the pathogenesis of eczema and AD [10-12]. researchers and their colleagues may bring this understanding closer and could help lead to better treatments. In a paper published online in Nature, the team reports that a toxin produced by the common bacteria Staphylococcus aureus-causes immune-system cells in the skin to react in a way that produces eczema-like rashes. The release of the molecule, called delta toxin, by staph bacteria caused immunerelated mast cells in the skin to release tiny granules that cause inflammation. These studies identified δ-toxin as a potent inducer of mast cell degranulation and suggest a mechanistic link between S. aureus colonization and allergic skin disease [13]. These studies show that there is a strong link between eczema and bacterial infection especially S. aureus.
In our previous studies conducted for Ochrocarpus longifolius extract we had observed significant Anti-bacterial activity against S. aureus. MIC value as low as 1-5 mcg/ml was obtained for different extracts of Ochrocarpus longifolius. The reduction in erythema and edema observed in our clinical study may be due to the significantanti-inflammatory activity of Ochrocarpus longifolius extract exhibited by inhibition of TNF-α. Similarly, the anti-bacterial effect may have been responsible for reduction in oozing and eventually lead to the increase in healing activity. Staphylococcus delta-toxin may play a role in excessive pruritus and the use of Eczero® cream causing inhibition of bacterial colony must have caused the reduction in pruritus significantly. The VAS score for pruritus was assessed as the immediate complaint of eczema and it showed significant reduction of pruritus [F (5, 38) = 9.68, p = <0.0001] over application period of 28 days. Thus we may postulate that the clinical findings support our lab results with significant reduction observed in signs and symptoms of eczema in the studied patients.
Mast cells (MC) being important source of TNF-α influences the inflammatory response found in Eczema. In order to evaluate this hypothesis, we have conducted an in-vitro study with hpBMC assay. The anti-inflammatory potential of Ochrocarpus longifolius extract was confirmed using a screening model of inflammation, specifically directed towards inhibition of TNF- Α. It was observed that the extract showed a dose-dependent inhibition of LPS-induced TNF- Α release in hPBMCs after 5 h incubation. The clinical study supports that the transdermal absorption of Eczoro® cream containing extract of Ochrocarpus longifolius, may be effective in preventing the allergic and inflammatory response by inhibiting the mast cell degranulation. Also, the standardized extract of Ochrocarpus longifolius may have acted as a potential anti-inflammatory agent preventing the redness, itching and the thickness of the skin. The wound healing effect may restore the skin texture and reduce the lesion formation
Eczero® cream may play a significant role in treatment regimen of eczema patients. Many of the available topical treatments have reported adverse effects associated with it. Some of the topical therapies like corticosteroids and calcineurin inhibitors (i.e. pimecrolimus and tacrolimus) may have serious side-effects in the eczema patients. Prolonged use of topical corticosteroids can result in telangiectasias, increased hair, skin tears, easy bruising, poor wound healing, acne and rosacea, and thinning/atrophic changes, which can be permanent [14-16]. Topical corticosteroids can also produce systemic effects including adrenal suppression, particularly when higher potency preparations are used for long periods on large surface areas or more permeable areas of the skin [17]. Topical preparations of calcineurin inhibitors may cause stinging in some of the sensitive patients. The US FDA label for topical calcineurin inhibitors includes a “black box” warning regarding a theoretical risk for skin cancers and lymphoma [18-19]. Being of a natural origin Eczero® cream in the present study demonstrated negligible side-effects and hence presents a positive option for eczema therapy.
Conclusion
The present study demonstrates the efficacy of Eczero® cream-a plant based topical formulation in reducing the signs and symptoms of mild to moderate eczema in the studied patients. Eczero® cream significantly reduced the appearance of lesions as well as associated symptoms like itching, scaling, and redness. Eczero®cream was welltolerated and safe with no serious adverse events observed. The dual property of cream-its anti-bacterial and anti-inflammatory activity presents a perfect combination for the treatment of eczema. A further study in larger population will help to potentiate the clinical effectiveness of this topical formulation.
References
- Brown S, Reynolds NJ (2006) Atopic and non-atopic eczema. BMJ 332: 584-588.
- Pastore S, Mascia F, Giustizieri ML, Giannetti A, Girolomoni G (2001) Pathogenetic mechanisms of atopic dermatitis. In: Gorski A, Krotkiewski H, et al. (Eds), Inflammation. Springer, Dordrecht, Switzerland, pp. 109-122.
- Pastar Z, Lipozencic J, Ljubojevic S (2005) Etiopathogenesis of atopic dermatitis--an overview. Acta Dermatovenerol Croat 13: 54-62.
- Kabashima K (2013) New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci 70: 3-11.
- Bamford JT, Ray S, Musekiwa A, van Gool C, Humphreys R, et al. (2013) Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev: CD004416.
- Ambasta SP (1986) The useful plants of India. CSIR, New Delhi, India, pp. 918.
- Chopra RN, Nayar SL, Chopra IC (1956) Glossary of medicinal plants of India. CSIR, New Delhi, India, pp. 330.
- Deng Y, Nicholson RA (2005) Antifungal properties of surangin B, a coumarin from Mammea longifolia. Planta Med 71: 364-365.
- Salkar K, Chotalia C, Salvi R (2018) Antibacterial activity of Ochrocarpus longifolius extract against S. aureus and MRSA strains. WJPLS 4: 161-165.
- Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, et al. (2006) Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicenter randomized controlled trial. Br J Dermatol 155: 680-687.
- 11.Higaki S, Morohashi M, Yamagishi T, Hasegawa Y (1999) Comparative study of Staphylococci from the skin of atopic dermatitis patients and from healthy subjects. Int J Dermatol 38: 265-269.
- Goh CL, Wong JS, Giam YC (1997) Skin colonization of Staphylococcus aureus in atopic dermatitis patients seen at the National Skin Centre, Singapore. Int J Dermatol 36: 653-657.
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, et al. (2013) Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature 503: 397-401.
- Bleehen SS, Chu AC, Hamann I, Holden C, Hunter JA, et al. (1995) Fluticasone propionate 0.05% cream in the treatment of atopic eczema: a multicenter study comparing once-daily treatment and once-daily vehicle cream application versus twice-daily treatment. Br J Dermatol 133: 592-597.
- Katz HI, Prawer SE, Mooney JJ, Samson CR (1989) Preatrophy: covert sign of thinned skin. J Am Acad Dermatol 20: 731-735.
- Furue M, Terao H, Rikihisa W, Urabe K, Kinukawa N, et al. (2003) Clinical dose and adverse effects of topical steroids in daily management of atopic dermatitis. Br J dermatol 148: 128-133.
- Lam LH, Sugarman JL (2016) Adrenal suppression with chronic topical corticosteroid use in psoriasis patients. J Drugs Dermatol 15: 945-948.
- Draelos ZD (2008) Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin 24: 985-994.
- Mandelin J, Remitz A, Virtanen H, Reitamo S (2010) One-year treatment with 0.1% tacrolimus ointment versus a corticosteroid regimen in adults with moderate to severe atopic dermatitis: a randomized, double-blind, comparative trial. Acta Derm Venereol 90: 170-174.