Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
*Address for Correspondence: Joaquin J. Jimenez, MD., Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, 1600 NW 10th Avenue, RMSB, Room 2023A, Miami, FL 33136 USA, Tel: 305-243- 6586; Fax: 305-243-6191; E-mail: j.jimenez@med.miami.edu
Citation: Villasante AC, Petit VA, Yin NC, Elgart GW, Schachner LA, et al. A Comparison of Methods of Anagen Synchronization in the Adult Rat Model of Chemotherapy-Induced Alopecia. J Clin Investigat Dermatol. 2015;2(3): 3.
Copyright © Jimenez et al.This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical & Investigative Dermatology | ISSN: 2373-1044 | Volume: 2, Issue: 3
Submission: 09 January 2015| Accepted: 28 January 2015 | Published: 31 January 2015
Reviewed & Approved by: Dr. Jeff Donovan, Assistant Professor, University of Toronto, USA
A Comparison of Methods of Anagen Synchronization in the Adult Rat Model of Chemotherapy-Induced Alopecia
A.C. Villasante11, V.A. Petit11, N.C. Yin1, G.W. Elgart1, L.A. Schachner1 and J.J. Jimenez1,2*
- 1Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, USA
- 2Department of Biochemistry and Molecular Biology, University of Miami Miller School of Medicine, USA
*Address for Correspondence: Joaquin J. Jimenez, MD., Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, 1600 NW 10th Avenue, RMSB, Room 2023A, Miami, FL 33136 USA, Tel: 305-243- 6586; Fax: 305-243-6191; E-mail: j.jimenez@med.miami.edu
Citation: Villasante AC, Petit VA, Yin NC, Elgart GW, Schachner LA, et al. A Comparison of Methods of Anagen Synchronization in the Adult Rat Model of Chemotherapy-Induced Alopecia. J Clin Investigat Dermatol. 2015;2(3): 3.
Copyright © Jimenez et al.This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical & Investigative Dermatology | ISSN: 2373-1044 | Volume: 2, Issue: 3
Submission: 09 January 2015| Accepted: 28 January 2015 | Published: 31 January 2015
Reviewed & Approved by: Dr. Jeff Donovan, Assistant Professor, University of Toronto, USA
Abstract
Background: Chemotherapy-induced alopecia is a common, psychosocially distressing side effect of cancer treatment for which preventative therapies are lacking. Several animal models have been developed to study CIA, including adult murine models. In order to induce CIA in adult models, hair follicles must be synchronized into anagen. The present study seeks to investigate which method of anagen synchronization yields the most representative model of CIA; no study thus far has compared these methods.Methods: Biopsies were analyzed of adult rat skin treated with one of four methods of anagen synchronization: clipping, shaving, waxing, or plucking and an untreated control group. Twenty follicles were assessed at random from each animal in each group and the percentage of hair follicles in anagen V-VI was determined.
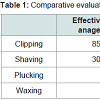
Results: There were significant differences in the histological and gross profiles of clipping, shaving, waxing, and plucking. Clipping produced the most robust anagen, with 85% of hair follicles in anagen V-VI, while shaving was found to have 30% of follicles in anagen V-VI. Waxing, plucking, and control had no hair follicles in anagen V-VI. Furthermore, shaving, waxing, and plucking demonstrated noticeable gross skin trauma, with waxing resulting in the most severe.
Conclusions: Clipping is the preferred method of anagen synchronization in the adult rat model for CIA, as it produces the most robust anagen and has no evidence of gross skin trauma, decreasing the likelihood of confounding results from traumatic hair removal. The next best method is shaving, followed by plucking, and ending with waxing.
Keywords
Chemotherapy; Anagen; Alopecia; Shaving; Clipping; Waxing; PluckingAbbreviations
CIA: Chemotherapy-induced Alopecia; HF: Hair FollicleIntroduction
Chemotherapy-induced alopecia (CIA), occurring in approximately 65% of chemotherapy patients, is experienced by many as a severely disturbing event [1,3]. As of yet, no preventative therapy for CIA has shown to completely protect patients from alopecic chemotherapy [1,4]. Several animal models have been developed to screen preventative therapies for CIA. In order for an animal model to be susceptible to CIA, its hair follicles (HFs) must be rapidly proliferating in anagen. The first CIA murine model was the albino Spraque Dawley neonatal rat in which chemotherapy was administered before postnatal day 14, up until which time its HFs are synchronized in anagen [5,6]. The next murine model developed was the adult mouse, using 6- to 8-week-old C57/BL6 mice [7]. Synchronization into anagen was required prior to the administrationof chemotherapy because most HFs on 6- to 8-week-old mice are in telogen or catagen [6].Anagen synchronization in the adult mouse model was accomplished by removal of telogen hairs via waxing [7]. Waxing, however, has been shown in mice to induce wounding trauma and results in the generation of fewer hair shafts, thinner hair fibers, and lower melanin content per hair shaft [8]. When the adult pigmented Long-Evans (LE) rat model was developed, clipping was instead used to induce anagen [9]. Adult LE rats are clipped on day 21 and chemotherapy is administered between day 36 and day 38 [9]. Given the potentially confounding features of traumatic hair removal, we sought to compare the following methods of anagen synchronization in adult LE rat skin: clipping, shaving, waxing and plucking.Materials and Methods
All animal care and use procedures were approved by the University of Miami Institutional Animal Care and Use Committee. LE rats were purchased from Charles River Laboratories, Wilmington, MA, USA and were kept under standard conditions. Rats were anesthesized with ketamine/xylazine (50 mg/kg and 5 mg/kg, Sigma- Aldrich, St. Louis, MO, USA) prior to anagen synchronization. Animals were euthanized according to IACUC procedures.Twenty-one day old rats were randomized into five cohorts, I to V, of ten rats each. Hair shafts were removed by applying the following treatments to the dorsal back skin: (I) clipping, (II) shaving, (III) waxing, and (IV) plucking. In group I, hair was removed with an electric clipper, as described [9]. Group II animals were covered in shaving cream before removing hair by applying a razor close to the skin. Caution was taken not to abrade the skin with the razor or the electric clipper. In group III, a melted wax/rosin mixture was applied to the skin and was peeled off after hardening, as described [6,7]. In group IV, hair was removed with a tweezer. An untreated group (V) was used for weight and histological comparison.Skin samples were obtained for histological analysis on postnatal day 36, fifteen days after the initial hair removal treatment. Twenty hair follicles from every animal in each group were analyzed at random to determine the percentage of hair follicles in anagen V-VI. Dorsal skin biopsies were fixed in 10% formalin, dehydrated and embedded in paraffin wax, before being cut into 5 μm sections and stained with hematoxylin and eosin. Images were captured using a microscope with a 4x objective (Observer D1; Carl Zeiss Microimaging Inc., Thornwood, NY, USA).Results
Rats demonstrated gross evidence of hair removal immediately after respective methods of anagen synchronization on day 21 (figures not shown). Grossly, the waxed group showed substantial scabbing of dorsal skin and the shaved and plucked groups showed minimal scabbing. The clipped group showed no signs of gross skin trauma.There were significant differences in the histological profiles of clipping, shaving, waxing, and plucking on day 36. Clipping resulted in a robust anagen, with 85% (170/200) of HFs displaying features of anagen V-VI: prominent hair bulbs, HFs penetrating the subcutaneous fat, and thick hair shafts protruding above the epidermis(Figure 1b and Figure 2). In the shaved group, 30% (60/200) of HFs were found in anagen V-VI (Figure 1c and Figure 2). Waxed, plucked, and control groups had no HFs in anagen V-VI; HFs in these groups did not penetrate the subcutaneous layer, hair bulbs were less prominent, and hair shafts appeared thinner (Figure 1a,1d,1e and Figure 2). Using Fisher’s exact test, the difference between clipped and shaved groups was statistically significant (p<0.01). The difference between the clipped group and the waxed or plucked groups was also significant (p<0.01). Finally, the results of the shaved group were also significantly different from the waxed or plucked (p<0.01).Taking into account both effectiveness of inducing anagen, which is demonstrated in (Figure 2), and the risk of skin trauma, clipping is the superior method as it has the highest percentage of follicles in anagen V-VI and has no evidence of gross skin trauma. The next best method is shaving, followed by plucking, and ending with waxing. These comparisons are demonstrated in Table 1.
Discussion
Adult murine models of CIA require anagen synchronization in order to best represent the human scalp. Anagen synchronization in murine models is accomplished by hair removal prior to the administration of chemotherapy. Previously, both waxing in the adult mouse and clipping in the adult rat models have resulted in effective induction of CIA [7,9]. Here, we found that fifteen days post clipping in the adult rat model resulted in a robust anagen with 85% of HFs in anagen V-VI and with normal-looking skin and HFs. Shaving resulted in a moderately robust anagen with 30% of follicles in V-VI. However, with shaving there is a risk of accidental wound-induction by nicking, even with careful technique. Waxing and plucking resulted in no follicles in anagen V-VI. At this point in time, waxing and plucking may also lead to skin irritation and erosion. We used the fifteen-day post hair removal time point for hair analysis comparison because from the literature and our previous experience it is the optimal time point to induce chemotherapy-induced alopecia. The traumatic effects of waxing, plucking, and shaving in CIA murine models may confound results. Clipping is, therefore, the preferred method of anagen synchronization in the adult rat model for CIA because it produces the most robust anagen, it does not traumatize HFs or skin, and it is the method least stressful to the animals.References
- Villasante AC, Herskovitz I, Mauro LM, Jimenez JJ (2014) Chemotherapy-induced alopecia. J Clin Invest Dermatol 2: 9.
- Trueb RM (2010) Chemotherapy-induced hair loss. Skin Therapy Lett 15: 5-7.
- Chon SY, Champion RW, Geddes ER, Rashid RM (2012) Chemotherapy-induced alopecia. J Am Acad Dermatol 67: e37-47.
- Yeager CE, Olsen EA (2011) Treatment of chemotherapy-induced alopecia. Dermatol Ther 24: 432-442.
- Hussein AM, Jimenez JJ, McCall CA, Yunis AA (1990) Protection from chemotherapy-induced alopecia in a rat model. Science 249: 1564-1566.
- Müller-Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, et al. (2001) A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 117: 3-15.
- Paus R, Handjiski B, Eichmuller S, Czarnetzki BM (1994) Chemotherapy-induced alopecia in mice: induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol 144: 719-734.
- Plonka PM, Michalczyk D, Popik M, Handjiski B, Paus R (2008) Electron paramagnetic resonance (EPR) spectroscopy for investigating murine telogen skin after spontaneous or depilation-induced hair growth. J Dermatol Sci 49: 227-240.
- Wikramanayake TC, Amini S, Simon J, Mauro LM, Elgart GW, et al. (2012) A novel rat model for chemotherapy-induced alopecia. Clin Exp Dermatol 37: 284-289.