Journal of Cardiobiology
Download PDF
Special Issue: Cardiac Surgery and its OutcomesReview Article
*Address for Correspondence: Jonathan Grinstein, MD, Section of Cardiology, Department of Medicine, University of Chicago Medical Center, 5841 South Maryland Ave, MC 6092 Chicago, IL, 60637, USA, E-mail: Jonathan.Grinstein@uchospitals.edu
Citation: Grinstein J, Hofmann Bowman MA, Fedson S. Ventricular Assist Devices in Advanced Heart Failure: Exploring the Technology’s Current Utilization, Limitations and Future Directions. J Cardiobiol. 2014;S(1): 7.
Copyright © 2014 Grinstein J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Special Issue 2
Submission: 22 August, 2014 | Accepted: 12 September, 2014 | Published: 16 September, 2014
Editor:Dr. Anas Sarraj Asil, Associate Professor of Cardiovascular Surgery Universidad Autónoma de Madrid, Spain
Given the fundamental limitations of pulsatile assist devices, pump designers focused on developing continuous flow assist devices with rotary impellers. These comprise a single moving part and have enhanced durability. There are currently two dominant continuous flow devices on the market: the HeartMate II (Thoratec, Pleasanton, CA) and the HeartWare HVAD (HeartWare, Framingham, MA). The HeartMate II, a continuous flow device with an axial flow pattern was shown to be an effective bridge to transplantation in the HeartMate II BTT trial. In this trial, 75% of patients successfully received transplantation, recovered or continued to be eligible for transplantation at 6 months after implantation [8]. The HeartMate II DT trial showed that in patients ineligible for transplantation, the HeartMate II was superior to the HeartMate XVE with a 62% increased rate of survival free of disabling stroke or reoperation with the HeartMate II [9]. The FDA approved the HeartMate II for BTT in 2008 and for DT in 2010. The HeartWare HVAD is a continuous flow device with a centrifugal flow pattern that is small enough to allow for intrapericardial implantation. The ADVANCE trial found the HeartWare to be non-inferior to commercially available durable LVADs [10]. In 2012, the HeartWare was approved for BTT. The ENDURANCE trial is currently assessing the use of HeartWare for DT with a head to head comparison with the HeartMate II [11].
Following FDA approval of the first continuous flow LVAD in 2008, the field has made a remarkable shift from pulsatile to continuous flow devices. Continuous flow devices now account for 100% of implanted LVADs in patients receiving DT and over 95% of patients receiving mechanical circulatory support [12]. Similarly, there has been a shift from BTT to DT over the years. In 2006-2007, 42.4% of VAD patients were listed for transplant and 14.7% of patients received devices as a final destination. In 2011-2013, 21.7% of patients were listed for transplant and 41.6% of implanted LVADs were for DT (Figure 1B) [12]. Currently there are numerous FDAapproved devices with varied pump designs (Table 1). Devices can be continuous flow or pulsatile, intracorporeal or paracorporeal, durable or non - durable (temporary). Lastly, devices can assist the failing ventricle (VADs) or replace the heart in the form of a total artificial heart (TAH). Currently, 97% of implanted devices are continuous flow, intracorporeal LVADs [12].
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile was developed to better characterize and stratify patients with advanced heart failure. Patients with NYHA functional class IIIb and IV heart failure with symptoms limiting daily activity are grouped into seven profiles (INTERMACS profiles 1-7), depending on the clinical severity of the patient [15]. Traditionally, VAD implantation has been reserved for the sickest patients who are inotrope-dependent (INTERMACS 1-3). Dating back to 2008, 81% of patients receiving LVAD or BiVAD implantation have been inotrope dependent (Figure 2A) [12]. Recently, there has been a shift towards implanting ventricular assist devices in more stable patients in an attempt to avoid the morbidity and mortality of performing surgery in the critically ill. Boyle et al. showed that patients with ambulatory advanced heart failure (INTERMACS Profiles 4-7) had better survival and shorter lengths of stay in the hospital [16]. Accordingly, recent INTERMACS trends show a decrease in the number of patients with more advanced disease receiving LVAD or BiVAD support (Figure 2B) [12]. There is currently an ongoing NIH sponsored trial (REVIVEIT) which is looking at earlier implantation in the INTERMACS 4-5 patient [17].
Not all patients with advanced heart failure are suitable candidates for mechanical circulatory support. Comorbid medical conditions, operative risk, and psychosocial factors must all be considered before offering device implantation. Patients in acute cardiogenic shock (INTERMACS I) who are deemed to have a low likelihood of ventricular recovery who have irreversible end-organ damage despite temporary device support should be considered for MCS (Class IIa, LOA C) [13].Similarly, inotrope-dependent patients (INTERMACS II and III) should be considered for MCS given their high one-year mortality rate (Class IIa, LOA B) [13].
VAD thrombosis is defined as the formation of a blood clot in the VAD rotor, inlet cannula or outlet cannula. While the initial incidence of device thrombosis was thought to be 2%, the rate of reported VAD thrombosis has increased over the past decade with a current prevalence of device thrombosis thought to be over 8% [22]. Patient-specific factors, such as active infection, atrial tachyarrhythmias and non-compliance with anti-platelet or anticoagulation therapy, predispose an individual to device thrombosis. Furthermore, management-related factors such as the position and integrity of the inlet and outlet cannula, permissive subtherapeutic anticoagulation or platelet inhibition in the setting of acute bleeding, and reduced pump flow, all increase the rate of thrombosis [23].
VAD thrombosis can clinically manifest in a myriad of ways. Classically, patients will present with sustained power surges on their device accompanied by signs and symptoms of pulmonary congestion. Often, patients have dark-colored urine resulting from severe hemolysis and hemoglobinuria. Lactate dehydrogenase (LDH) and plasma-free hemoglobin are released with hemolysis and elevation of LDH > 3 times the upper limit of normal or a plasma-free hemoglobin level > 40 mg/dl are highly suggestive of device thrombosis [23]. A ramp test which consists of a series of echocardiographic images of the left-ventricular end-diastolic diameter (LVEDD), aortic valve opening and valvular function with changing LVAD speeds can similarly aid in the diagnosis of VAD thrombosis. A ramp test that shows a failure of the LVEDD to decrease despite increasing LVAD speeds is highly suggestive of device thrombosis [24]. While changes in LVEDD can be seen on ramp studies with axial flow devices, abrupt changes in VAD setting might not result in changes in 2-D echocardiographic assessment of LVEDD in centrifugal flow pumps.
In these cases, the opening of the aortic valve, and changes in any mitral regurgitation can be used to determine VAD function.
With device thrombosis, VAD performance decreases and patients often require inodilators or inotropes to manage clinical signs of heart failure. Initial management with IV heparin leads to resolution of small thromboses in many cases. The use of intravenous direct thrombin inhibitors and glycoprotein IIb/IIIa inhibitors has been attempted with success in a handful of case series [25,26]. If thrombosis persists, a trial of tissue plasminogen activator (tPA) can be attempted before surgical LVAD pump exchange in patients with HeartWare HVAD devices [23]. For patients, with HeartMate II devices, tPA has been less uniformly effective although catheterdirected lytics have been shown to be effective in select patients [27]. However, often VAD exchange is needed, with actuarial 1 year freedom from repeat device exchange approaching 90% [28]. If resolution of device thrombosis is achieved, long-term preventative strategies often involve higher INR targets and the addition of a second anti-platelet agent such as clopidogrel or dipyridamole, to aspirin therapy.
Bleeding
The HeartWare MVAD pump is a continuous axial flow pump that is approximately one third the size of the current HVAD pump [47,48]. With continued miniaturization of the technology, future LVAD implantation may be achievable with minimally or less invasive surgery allowing for decreased perioperative morbidity and mortality. The small pump profile together with new cannula designs may allow for the avoidance of a full sternotomy and elimination of cardiopulmonary bypass through an apical approach [49]. The HeartMate III is an experimental centrifugal pump that is currently in the early investigational stages. The HeartMate III has several design elements aimed at reducing the thrombosis rate. The device’s textured surface provides a tissue to blood interface that is felt to be less thrombogenic than current models. Additionally, the HeartMate III incorporates pulsed technology that assists with pump wash out and intermittent aortic valve opening, with the goal to minimize thrombus formation and de novo aortic insufficiency [50]. These smaller devices may also find a role in the management of right ventricular failure and pulmonary artery hypertension. Concurrent with pump redesign, considerable research is underway in technologies that allow for fully implantable systems, thereby reducing or eliminating drivelineassociated infections. Transcutaneous energy transfer (TET) is a technology that allows for periodic and non-invasive recharging of an implanted battery. TET uses induction-heating which creates an electromagnetic current between coils located inside and outside of the body [51]. With continued advancements in design, device efficiency, reliability, durability and cost, LVADs will become even more readily available to patients with advanced heart failure.
Ventricular Assist Devices in Advanced Heart Failure: Exploring the Technology’s Current Utilization, Limitations and Future Directions
Jonathan Grinstein*, Marion A. Hofmann Bowman and Savitri Fedson
- Section of Cardiology, Department of Medicine, University of Chicago, Chicago, IL, USA
*Address for Correspondence: Jonathan Grinstein, MD, Section of Cardiology, Department of Medicine, University of Chicago Medical Center, 5841 South Maryland Ave, MC 6092 Chicago, IL, 60637, USA, E-mail: Jonathan.Grinstein@uchospitals.edu
Citation: Grinstein J, Hofmann Bowman MA, Fedson S. Ventricular Assist Devices in Advanced Heart Failure: Exploring the Technology’s Current Utilization, Limitations and Future Directions. J Cardiobiol. 2014;S(1): 7.
Copyright © 2014 Grinstein J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Special Issue 2
Submission: 22 August, 2014 | Accepted: 12 September, 2014 | Published: 16 September, 2014
Editor:Dr. Anas Sarraj Asil, Associate Professor of Cardiovascular Surgery Universidad Autónoma de Madrid, Spain
Keywords
Congestive heart failure; Ventricular assist devicesAbbreviations
VAD: Ventricular Assist Device; LVAD: Left Ventricular Assist Device; RVAD: Right Ventricular Assist Device; BiVAD: Biventricular Assist Device; BTT: Bridge to Transplant; BTR: Bridge to Recovery; DT: Destination Therapy; BTC: Bridge to Candidacy; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; SHFM: Seattle Heart Failure Modes; HFSS: Heart Failure Survival; Score AI: Aortic InsufficiencyIntroduction
An estimated 5.1 million adults in the United States have heart failure with 5% of patients having end-stage or advanced heart failure that is refractory to traditional medical therapy [1,2]. Heart transplant remains the best therapeutic and durable option for those patients who are eligible. Median survival following transplantation is 11 years for all-comers and 13 years among those surviving the first year [3]. Survival after transplantation has improved over the years with an unadjusted 1-year survival of 84% and a 5 year survival for those surviving the first year of 85% [3]. Unfortunately, there is a supply-demand mismatch for viable donor hearts with 4026 patients in the United States currently waiting for transplant and only 2531 transplants in 2013 (Figure 1A) [4]. In the current environment of organ allocation limitations and an escalating population of transplant-ineligible patients, ventricular assist devices (VADs) have emerged as a viable option for durable hemodynamic support. VADs unload the failing left ventricle and maintain cardiac output and organ perfusion while at the same time, rest the burdened ventricle allowing for recovery. VADs can be implanted in the left ventricle (LVAD), right ventricle (RVAD) or both ventricles simultaneously (BiVAD). Whereas RVADs and BiVADs are currently only approved for temporary support during recovery, LVADs are indicated for three general purposes: 1) Bridge to recovery, 2) Bridge to transplant, and 3) Destination therapy. In selected patients with a reversible cardiac insult such as after myocardial infarction, postcardiotomy shock or fulminant myocarditis, VADs can be used as a bridge to recovery (BTR) and then removed after the native heart has recovered. For patients who fail to recover or patients with refractory congestion or progressive end-organ dysfunction, LVADs can be used to improve the quality of life. In transplant-eligible patients who are anticipated to have a long wait time or who otherwise have a poor quality of life, LVADs can be used as a bridge to transplant (BTT). For transplant-ineligible patients with advanced age or multiple comorbidities, LVADs can be used for durable, indefinite support as a destination therapy (DT). For patients who are initially ineligible for transplant due to end-organ dysfunction, nutritional deficiencies or undesirable psychosocial conditions who may become eligible in the future with improvement in the clinical status, LVADs are functionally used as a bridge to candidacy (BTC) [5].
History of Ventricular Assist Devices
The first ventricular assist device was reported in 1963 by Liotta et al. as a bridge to recovery [6]. The VAD consisted of a pneumatic pump with a valved conduit connecting the left atrium and aorta. The first pulsatile, electrical LVAD was successfully implanted as a bridge to transplant in 1984, and was followed by the FDA approval of LVADs for BTT in 1990 [5]. The landmark REMATCH trial in 2001 was the first to show that LVADs as DT was superior to medical therapy for transplant-ineligible patients. REMATCH found a 48% relative risk reduction in mortality with the pulsatile HeartMate XVE LVAD compared to medical therapy alone in patients who were not eligible for heart transplantation [7]. In 2003, the FDA approved the HeartMate XVE for destination therapy (DT) for transplant ineligible patients. Unfortunately, pulsatile assist devices were prone to early device malfunction owing to multiple moving parts, bearings and friction. Furthermore, the size of pulsatile assist device was prohibitive for many patients.Given the fundamental limitations of pulsatile assist devices, pump designers focused on developing continuous flow assist devices with rotary impellers. These comprise a single moving part and have enhanced durability. There are currently two dominant continuous flow devices on the market: the HeartMate II (Thoratec, Pleasanton, CA) and the HeartWare HVAD (HeartWare, Framingham, MA). The HeartMate II, a continuous flow device with an axial flow pattern was shown to be an effective bridge to transplantation in the HeartMate II BTT trial. In this trial, 75% of patients successfully received transplantation, recovered or continued to be eligible for transplantation at 6 months after implantation [8]. The HeartMate II DT trial showed that in patients ineligible for transplantation, the HeartMate II was superior to the HeartMate XVE with a 62% increased rate of survival free of disabling stroke or reoperation with the HeartMate II [9]. The FDA approved the HeartMate II for BTT in 2008 and for DT in 2010. The HeartWare HVAD is a continuous flow device with a centrifugal flow pattern that is small enough to allow for intrapericardial implantation. The ADVANCE trial found the HeartWare to be non-inferior to commercially available durable LVADs [10]. In 2012, the HeartWare was approved for BTT. The ENDURANCE trial is currently assessing the use of HeartWare for DT with a head to head comparison with the HeartMate II [11].
Following FDA approval of the first continuous flow LVAD in 2008, the field has made a remarkable shift from pulsatile to continuous flow devices. Continuous flow devices now account for 100% of implanted LVADs in patients receiving DT and over 95% of patients receiving mechanical circulatory support [12]. Similarly, there has been a shift from BTT to DT over the years. In 2006-2007, 42.4% of VAD patients were listed for transplant and 14.7% of patients received devices as a final destination. In 2011-2013, 21.7% of patients were listed for transplant and 41.6% of implanted LVADs were for DT (Figure 1B) [12]. Currently there are numerous FDAapproved devices with varied pump designs (Table 1). Devices can be continuous flow or pulsatile, intracorporeal or paracorporeal, durable or non - durable (temporary). Lastly, devices can assist the failing ventricle (VADs) or replace the heart in the form of a total artificial heart (TAH). Currently, 97% of implanted devices are continuous flow, intracorporeal LVADs [12].
Patient Selection
Before considering advanced heart failure options, a thorough evaluation and treatment of reversible causes of worsening heart failure, such as active coronary ischemia, valvular heart disease, arrhythmias or ongoing cardiotoxic insults, should be undertaken [13]. Additionally, evidence-based medical and device therapy such as beta blockers, ACE inhibitors, or angiotensin receptor antagonists, mineralocorticoid receptor antagonists and cardiac resynchronization therapy in appropriate patients should be optimized [14]. Given the superior long-term outcomes of heart transplantation, all patients being considered for mechanical circulatory support (MCS) should also be evaluated for transplant candidacy (Class I, LOA A) [13].The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile was developed to better characterize and stratify patients with advanced heart failure. Patients with NYHA functional class IIIb and IV heart failure with symptoms limiting daily activity are grouped into seven profiles (INTERMACS profiles 1-7), depending on the clinical severity of the patient [15]. Traditionally, VAD implantation has been reserved for the sickest patients who are inotrope-dependent (INTERMACS 1-3). Dating back to 2008, 81% of patients receiving LVAD or BiVAD implantation have been inotrope dependent (Figure 2A) [12]. Recently, there has been a shift towards implanting ventricular assist devices in more stable patients in an attempt to avoid the morbidity and mortality of performing surgery in the critically ill. Boyle et al. showed that patients with ambulatory advanced heart failure (INTERMACS Profiles 4-7) had better survival and shorter lengths of stay in the hospital [16]. Accordingly, recent INTERMACS trends show a decrease in the number of patients with more advanced disease receiving LVAD or BiVAD support (Figure 2B) [12]. There is currently an ongoing NIH sponsored trial (REVIVEIT) which is looking at earlier implantation in the INTERMACS 4-5 patient [17].
Not all patients with advanced heart failure are suitable candidates for mechanical circulatory support. Comorbid medical conditions, operative risk, and psychosocial factors must all be considered before offering device implantation. Patients in acute cardiogenic shock (INTERMACS I) who are deemed to have a low likelihood of ventricular recovery who have irreversible end-organ damage despite temporary device support should be considered for MCS (Class IIa, LOA C) [13].Similarly, inotrope-dependent patients (INTERMACS II and III) should be considered for MCS given their high one-year mortality rate (Class IIa, LOA B) [13].
Several models and diagnostic tests are available to help determine when it is appropriate to consider MCS in ambulatory patients who are not inotrope dependent (INTERMACS 4-7). Traditional models incorporate both indices of cardiac performance as well as endorgan function to determine prognosis. The Seattle Heart Failure Model (SHFM) incorporates 20 clinical and laboratory variables and increasing scores with this model is associated with decreasing eventfree survival in ambulatory patients awaiting transplantation [18]. Similarly, a modified SHFM which incorporates additional variables of IABP use, ventilator-dependence and inotrope-dependence successfully predicted outcomes in medically-treated and LVADtreated cohorts [19]. The model does appear to underestimate risk in certain subgroups including African Americans, INTERMACS level 1 patients, and patients who subsequently require biventricular support [13]. An alternative model, the Heart Failure Survival Score (HFSS) includes 7 variables including resting heart rate, mean blood pressure, left ventricular ejection fraction, serum sodium concentration, presence of ischemic heart disease, QRS ≥ 120 ms, and peak VO2. The HFSS provides better prognostic value than metabolic exercise testing alone and has similar discriminatory value as the SHFM in patients referred for transplant [13,18]. There are limitations to both of these models when predicting prognosis, mostly because they were derived in an ambulatory population and do not apply to those in INTERMACS 1-3.
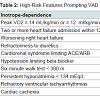
Cardiopulmonary exercise testing is well validated and routinely used in determining transplant or LVAD candidacy. Patients are generally considered to be candidates if their peak VO2 is ≤ 14 mL/kg/min or ≤ 12 mL/kg/min if on a beta blocker, or if their predicted peak VO2 is less than 50% predicted for age and sex [20]. Inotrope-dependency is another important variable to consider when considering advance heart failure options. Inotrope-dependent patients do poorly with medical therapy alone. In subgroup analysis of the REMATCH trial, randomization to LVAD led to a survival benefit in inotrope-dependent patients but not in those who were not on inotropes at baseline [21]. Additional high-risk features that are associated with increased mortality and should trigger mechanical circulatory support consideration include two or more heart failure hospitalizations in the past 12 months, cardiorenal syndrome limiting ACE or ARB utilization, hypotension limiting beta-blocker utilization, worsening right-heart failure from secondary pulmonary hypertension, 6-minute walk test < 300 m, diuretic refractoriness and persistent hyponatremia (Table 2) [5].
Cardiopulmonary exercise testing is well validated and routinely used in determining transplant or LVAD candidacy. Patients are generally considered to be candidates if their peak VO2 is ≤ 14 mL/kg/min or ≤ 12 mL/kg/min if on a beta blocker, or if their predicted peak VO2 is less than 50% predicted for age and sex [20]. Inotrope-dependency is another important variable to consider when considering advance heart failure options. Inotrope-dependent patients do poorly with medical therapy alone. In subgroup analysis of the REMATCH trial, randomization to LVAD led to a survival benefit in inotrope-dependent patients but not in those who were not on inotropes at baseline [21]. Additional high-risk features that are associated with increased mortality and should trigger mechanical circulatory support consideration include two or more heart failure hospitalizations in the past 12 months, cardiorenal syndrome limiting ACE or ARB utilization, hypotension limiting beta-blocker utilization, worsening right-heart failure from secondary pulmonary hypertension, 6-minute walk test < 300 m, diuretic refractoriness and persistent hyponatremia (Table 2) [5].
VAD Complications
ThrombosisVAD thrombosis is defined as the formation of a blood clot in the VAD rotor, inlet cannula or outlet cannula. While the initial incidence of device thrombosis was thought to be 2%, the rate of reported VAD thrombosis has increased over the past decade with a current prevalence of device thrombosis thought to be over 8% [22]. Patient-specific factors, such as active infection, atrial tachyarrhythmias and non-compliance with anti-platelet or anticoagulation therapy, predispose an individual to device thrombosis. Furthermore, management-related factors such as the position and integrity of the inlet and outlet cannula, permissive subtherapeutic anticoagulation or platelet inhibition in the setting of acute bleeding, and reduced pump flow, all increase the rate of thrombosis [23].
VAD thrombosis can clinically manifest in a myriad of ways. Classically, patients will present with sustained power surges on their device accompanied by signs and symptoms of pulmonary congestion. Often, patients have dark-colored urine resulting from severe hemolysis and hemoglobinuria. Lactate dehydrogenase (LDH) and plasma-free hemoglobin are released with hemolysis and elevation of LDH > 3 times the upper limit of normal or a plasma-free hemoglobin level > 40 mg/dl are highly suggestive of device thrombosis [23]. A ramp test which consists of a series of echocardiographic images of the left-ventricular end-diastolic diameter (LVEDD), aortic valve opening and valvular function with changing LVAD speeds can similarly aid in the diagnosis of VAD thrombosis. A ramp test that shows a failure of the LVEDD to decrease despite increasing LVAD speeds is highly suggestive of device thrombosis [24]. While changes in LVEDD can be seen on ramp studies with axial flow devices, abrupt changes in VAD setting might not result in changes in 2-D echocardiographic assessment of LVEDD in centrifugal flow pumps.
In these cases, the opening of the aortic valve, and changes in any mitral regurgitation can be used to determine VAD function.
With device thrombosis, VAD performance decreases and patients often require inodilators or inotropes to manage clinical signs of heart failure. Initial management with IV heparin leads to resolution of small thromboses in many cases. The use of intravenous direct thrombin inhibitors and glycoprotein IIb/IIIa inhibitors has been attempted with success in a handful of case series [25,26]. If thrombosis persists, a trial of tissue plasminogen activator (tPA) can be attempted before surgical LVAD pump exchange in patients with HeartWare HVAD devices [23]. For patients, with HeartMate II devices, tPA has been less uniformly effective although catheterdirected lytics have been shown to be effective in select patients [27]. However, often VAD exchange is needed, with actuarial 1 year freedom from repeat device exchange approaching 90% [28]. If resolution of device thrombosis is achieved, long-term preventative strategies often involve higher INR targets and the addition of a second anti-platelet agent such as clopidogrel or dipyridamole, to aspirin therapy.
Bleeding
Bleeding is another common complication after LVAD placement and accounts for 9% of the total mortality after LVAD insertion [29]. Major bleeding can lead to a reduction in LV preload and lead to suction events and hemodynamic instability. Similarly, reductions in RV preload with bleeding together with coronary hypoperfusion can lead to right ventricular failure. Post-operative bleeding rates continued to be extremely high despite improvements in surgical techniques. In the HeartMate II BTT trial, nearly one in three patients required reoperation for major bleeding and greater than 50% of patients required at least 2 units of blood in the first 30 days following implant [8]. In addition to the well-known standard risks of blood transfusions, including acquired infections and transfusionrelated lung injury, blood transfusions in VAD patients can lead to right-ventricular failure and alloimmunization leading to longer wait lists for subsequent cardiac transplant and cellular rejection after transplant [30].
Non-surgical bleeding, however, is the major hematologic problem of the continuous flow LVADs. As previously mentioned VAD patients should be on therapeutic doses of warfarin or other vitamin K antagonists and aspirin to prevent device thrombosis, increasing their propensity to bleed. Furthermore, almost every patient with a continuous flow LVAD develops acquired von Willebrand syndrome, a bleeding disorder characterized by structural defects of von Willebrand factor, caused by secondary shearing of high multimer polymers by the rotating impeller [31].
Additionally, it is hypothesized that the reduced pulsatility of continuous flow LVADs leads to hypoperfusion and neovascularization with friable vessels leading to arteriovenous malformations in the gastrointestinal tract and elsewhere which are prone to bleeding [31]. Reducing LVAD speeds in an attempt to increase pulsatility and reduce bleeding episodes in patients with previous gastrointestinal bleeding is often attempted but this comes at the expense of higher rates of VAD thrombosis as discussed above.
Initial management of significant bleeding should include temporary discontinuation of antiplatelet and anticoagulation therapy with reversal with fresh frozen plasma, cryoprecipitate or platelet products if the risk of fatal bleeding outweighs the risk of device thrombosis [32]. Octreotide, a somatostatin analogue known to inhibit angiogenesis, can be used in refractory GI bleeding, although its use failed to significantly reduce blood transfusion requirements, rebleeding rates, hospital length of stay or mortality [33]. Long acting octreotide may have some role for outpatient management of chronic GI bleeding. Diagnosis of GI bleeding begins with an upper and lower endoscopy but often involves double balloon enteroscopy and/or video capsule endoscopy to identify small-bowl sources of bleeding. If the site of bleeding is amenable to endoscopic intervention, cauterization, vessel clipping or injection of a vasoactive agent is often performed. Rarely, surgical resection of the involved bowel segment is indicated [31].
Non-surgical bleeding, however, is the major hematologic problem of the continuous flow LVADs. As previously mentioned VAD patients should be on therapeutic doses of warfarin or other vitamin K antagonists and aspirin to prevent device thrombosis, increasing their propensity to bleed. Furthermore, almost every patient with a continuous flow LVAD develops acquired von Willebrand syndrome, a bleeding disorder characterized by structural defects of von Willebrand factor, caused by secondary shearing of high multimer polymers by the rotating impeller [31].
Additionally, it is hypothesized that the reduced pulsatility of continuous flow LVADs leads to hypoperfusion and neovascularization with friable vessels leading to arteriovenous malformations in the gastrointestinal tract and elsewhere which are prone to bleeding [31]. Reducing LVAD speeds in an attempt to increase pulsatility and reduce bleeding episodes in patients with previous gastrointestinal bleeding is often attempted but this comes at the expense of higher rates of VAD thrombosis as discussed above.
Initial management of significant bleeding should include temporary discontinuation of antiplatelet and anticoagulation therapy with reversal with fresh frozen plasma, cryoprecipitate or platelet products if the risk of fatal bleeding outweighs the risk of device thrombosis [32]. Octreotide, a somatostatin analogue known to inhibit angiogenesis, can be used in refractory GI bleeding, although its use failed to significantly reduce blood transfusion requirements, rebleeding rates, hospital length of stay or mortality [33]. Long acting octreotide may have some role for outpatient management of chronic GI bleeding. Diagnosis of GI bleeding begins with an upper and lower endoscopy but often involves double balloon enteroscopy and/or video capsule endoscopy to identify small-bowl sources of bleeding. If the site of bleeding is amenable to endoscopic intervention, cauterization, vessel clipping or injection of a vasoactive agent is often performed. Rarely, surgical resection of the involved bowel segment is indicated [31].
Right heart failure
Right ventricular failure is a common occurrence following LVAD implantation, with an estimated incidence of 20-50%. Early RV failure leads to increased operative and post-operative mortality and morbidity and lengthens both intensive care and total hospital length of stay [34]. RV and LV function are exquisitely co-dependent and often LVAD implantation can have unpredictable effects on the RV. Successful LVAD implantation has the promise of reducing RV afterload by unloading the LV and decreasing left ventricular enddiastolic pressure and pulmonary vascular resistance. However, the increase flow generated by the LVAD will increase RV preload in the form of venous return back to the RV, and this may unmask preexisting RV dysfunction. Moreover, with severe LV unloading, the interventricular septum (IVS) bulges into the left ventricle, both as a result of the decrease in LV size and pressure but also from the increase in RV preload. RV function is significant influenced by the position of the IVS and the RV functions less efficiently when the IVS is markedly leftward. As a result, the remaining RV must work harder to compensate [34]. As the RV begins to fatigue, less blood is pumped from the RV to the LV and both native LV function and LVAD performance decline as LV preload decreases. This leads to a reduction in systemic mean arterial pressures which compromises coronary perfusion to the right ventricle. With increased RV workload and reduced RV coronary perfusion, the RV becomes ischemic which further worsens RV function. This interplay establishes a destructive cycle that further compromises RV function and is often difficult to recover from (Figure 3).
With significant RV dysfunction, inotropes with limited pulmonary vasodilation such as milrinone or dobutamine are added to support the RV. Caution must be taken to maintain adequate systolic pressures to maintain coronary perfusion to the RV and often a vasoactive inotrope such as norepinephrine or epinephrine must be added. Right sided-filling pressures should be maintained between 10 and 15 mm Hg and often loop diuretics, ultrafiltration or renal replacement therapy is necessary [34]. In patients with elevated pulmonary vascular resistance, specific pulmonary vasodilators such as phosphodiesterase type 5 inhibitors, inhaled nitric oxide or inhaled prostacyclins can be considered although prospective data supporting their use in this context is limited [35]. RV mechanical support with an RVAD is necessary in up to 11% of patients [36,37]. If RVAD support is necessary, early RVAD implantation at the time of LVAD surgery is better than delayed, provisional RVAD support with early RVAD implantation improving survival to transplant from 57% to 70% [38]. Chronic RV failure may develop over time, and management can include the use of oral phosphodiesterase-5 inhibitors or continuous inotrope infusions.
Right ventricular failure is a common occurrence following LVAD implantation, with an estimated incidence of 20-50%. Early RV failure leads to increased operative and post-operative mortality and morbidity and lengthens both intensive care and total hospital length of stay [34]. RV and LV function are exquisitely co-dependent and often LVAD implantation can have unpredictable effects on the RV. Successful LVAD implantation has the promise of reducing RV afterload by unloading the LV and decreasing left ventricular enddiastolic pressure and pulmonary vascular resistance. However, the increase flow generated by the LVAD will increase RV preload in the form of venous return back to the RV, and this may unmask preexisting RV dysfunction. Moreover, with severe LV unloading, the interventricular septum (IVS) bulges into the left ventricle, both as a result of the decrease in LV size and pressure but also from the increase in RV preload. RV function is significant influenced by the position of the IVS and the RV functions less efficiently when the IVS is markedly leftward. As a result, the remaining RV must work harder to compensate [34]. As the RV begins to fatigue, less blood is pumped from the RV to the LV and both native LV function and LVAD performance decline as LV preload decreases. This leads to a reduction in systemic mean arterial pressures which compromises coronary perfusion to the right ventricle. With increased RV workload and reduced RV coronary perfusion, the RV becomes ischemic which further worsens RV function. This interplay establishes a destructive cycle that further compromises RV function and is often difficult to recover from (Figure 3).
With significant RV dysfunction, inotropes with limited pulmonary vasodilation such as milrinone or dobutamine are added to support the RV. Caution must be taken to maintain adequate systolic pressures to maintain coronary perfusion to the RV and often a vasoactive inotrope such as norepinephrine or epinephrine must be added. Right sided-filling pressures should be maintained between 10 and 15 mm Hg and often loop diuretics, ultrafiltration or renal replacement therapy is necessary [34]. In patients with elevated pulmonary vascular resistance, specific pulmonary vasodilators such as phosphodiesterase type 5 inhibitors, inhaled nitric oxide or inhaled prostacyclins can be considered although prospective data supporting their use in this context is limited [35]. RV mechanical support with an RVAD is necessary in up to 11% of patients [36,37]. If RVAD support is necessary, early RVAD implantation at the time of LVAD surgery is better than delayed, provisional RVAD support with early RVAD implantation improving survival to transplant from 57% to 70% [38]. Chronic RV failure may develop over time, and management can include the use of oral phosphodiesterase-5 inhibitors or continuous inotrope infusions.
De novo aortic insufficiency
New onset, or de novo, aortic insufficiency (AI) is an increasingly recognized complication following LVAD implantation. The mechanism of AI associated with LVAD is incompletely understood, and it is believed that the lack of opening of the aortic valve due to the LVAD support together with altered hemodynamic force on the aortic valve leaflets promotes aortic valve commissural fusion and/or destruction of the leaflets [39]. The development of AI creates a redundant circulatory loop allowing for retrograde flow of blood back across the aortic valve, which reduces the efficiency of LVAD pumping. AI will develop in up to 30% of patients within three years of LVAD insertion; however, optimization of LVAD speed to allow for intermittent aortic valve opening prior to hospital discharge has been shown to significantly decrease the incidence of new onset AI [40]. It is unclear what effect these speed adjustments will have on thrombosis rates. Accordingly, future LVADs including the HeartWare MVAD and HeartMate III plan on incorporating intermittent low speed phases to promote intermittent aortic valve opening in an attempt to minimize these complications [40]. De novo AI can lead to clinical heart failure requiring aortic valve replacement, repair or closure and in some cases, urgent transplantation.
New onset, or de novo, aortic insufficiency (AI) is an increasingly recognized complication following LVAD implantation. The mechanism of AI associated with LVAD is incompletely understood, and it is believed that the lack of opening of the aortic valve due to the LVAD support together with altered hemodynamic force on the aortic valve leaflets promotes aortic valve commissural fusion and/or destruction of the leaflets [39]. The development of AI creates a redundant circulatory loop allowing for retrograde flow of blood back across the aortic valve, which reduces the efficiency of LVAD pumping. AI will develop in up to 30% of patients within three years of LVAD insertion; however, optimization of LVAD speed to allow for intermittent aortic valve opening prior to hospital discharge has been shown to significantly decrease the incidence of new onset AI [40]. It is unclear what effect these speed adjustments will have on thrombosis rates. Accordingly, future LVADs including the HeartWare MVAD and HeartMate III plan on incorporating intermittent low speed phases to promote intermittent aortic valve opening in an attempt to minimize these complications [40]. De novo AI can lead to clinical heart failure requiring aortic valve replacement, repair or closure and in some cases, urgent transplantation.
Infection
Up to one third of LVAD patients will develop a systemic infection with bacterial pathogens accounting for 87% off all infections followed by fungal pathogens [41]. VAD-related and VAD-specific infections are difficult to treat and are a major cause of mortality in the population. VAD-related infections are infections which are not unique to patients with VADs but requireunique consideration or management in patients with VADs such as infective endocarditis, bloodstream infections, and mediastinitis [42]. VAD-specific infections are infections that are specific to VAD patients and involve the device hardware or body surfaces containing them, including the driveline, pump, cannulas, hardware or device pocket [42]. Driveline infections are the most common source of infection in VAD patients with an incidence approaching 40% by three years following LVAD implantation [43]. Driveline infections may signify a deeper infection involving the pocket space or device hardware. Accordingly, computed tomography or ultrasound imaging for evidence of fluid collection around the VAD or pocket should be considered for all suspected driveline infections [42]. Pump and cannula infections are defined as proven, probable, possible or rejected based on a modified Duke’s criteria. Similar to the Duke’s criteria for infective endocarditis, a patient must have at least one microbiologic, histopathologic, echocardiographic or clinic criteria for the diagnosis of a pump or cannula infection to be suspected [42].
The clinical presentation in a patient with a VAD-specific or VADrelated infection can be quite variable, ranging from non-specific symptoms such as fatigue and lethargy to severe shock. Driveline and pocket infections can often be detected on physical exam as an area of purulence, fluctuance or erythema. Ultrasound and computed tomography is helpful in detecting VAD-specific infections around the pump or cannula although there is no great imaging modality for imaging the inside of the pump. Transthoracic or transesophageal echocardiogram can identify intracardiac masses near the inlet or outlet cannulas or evidence of infective endocarditis that might indirectly signify an internal pump infection. Initial empiric therapy for VAD-specific infections should cover both staphylococcal and pseudomonal species [44]. If clearance is possible, patients are usually placed on indefinite suppressive antibiotic therapy. When medical therapy is insufficient, surgical management with local debridement is sometimes successful but definitive management involves device explantation and reimplantation or transplantation. There are few data to support routine device exchange for chronic infections. Prevention is the key with respect to driveline infections. Education and meticulous care in driveline dressing changes is critical to prevent infections.
Up to one third of LVAD patients will develop a systemic infection with bacterial pathogens accounting for 87% off all infections followed by fungal pathogens [41]. VAD-related and VAD-specific infections are difficult to treat and are a major cause of mortality in the population. VAD-related infections are infections which are not unique to patients with VADs but requireunique consideration or management in patients with VADs such as infective endocarditis, bloodstream infections, and mediastinitis [42]. VAD-specific infections are infections that are specific to VAD patients and involve the device hardware or body surfaces containing them, including the driveline, pump, cannulas, hardware or device pocket [42]. Driveline infections are the most common source of infection in VAD patients with an incidence approaching 40% by three years following LVAD implantation [43]. Driveline infections may signify a deeper infection involving the pocket space or device hardware. Accordingly, computed tomography or ultrasound imaging for evidence of fluid collection around the VAD or pocket should be considered for all suspected driveline infections [42]. Pump and cannula infections are defined as proven, probable, possible or rejected based on a modified Duke’s criteria. Similar to the Duke’s criteria for infective endocarditis, a patient must have at least one microbiologic, histopathologic, echocardiographic or clinic criteria for the diagnosis of a pump or cannula infection to be suspected [42].
The clinical presentation in a patient with a VAD-specific or VADrelated infection can be quite variable, ranging from non-specific symptoms such as fatigue and lethargy to severe shock. Driveline and pocket infections can often be detected on physical exam as an area of purulence, fluctuance or erythema. Ultrasound and computed tomography is helpful in detecting VAD-specific infections around the pump or cannula although there is no great imaging modality for imaging the inside of the pump. Transthoracic or transesophageal echocardiogram can identify intracardiac masses near the inlet or outlet cannulas or evidence of infective endocarditis that might indirectly signify an internal pump infection. Initial empiric therapy for VAD-specific infections should cover both staphylococcal and pseudomonal species [44]. If clearance is possible, patients are usually placed on indefinite suppressive antibiotic therapy. When medical therapy is insufficient, surgical management with local debridement is sometimes successful but definitive management involves device explantation and reimplantation or transplantation. There are few data to support routine device exchange for chronic infections. Prevention is the key with respect to driveline infections. Education and meticulous care in driveline dressing changes is critical to prevent infections.
Neurologic events
Neurologic events associated with LVADs range from transientischemic attacks to cerebral embolic or hemorrhagic strokes. The incidences of these events have ranged from 6% to 9%, with lower incidences of ischemic stroke. There have been no differences noted in the incidence between DT and BTT candidates. Women appear to be at higher risk for all strokes [45,46]. Results from the 5th INTERMACS report reveal a 7% risk of stroke within the first two years after implantation [43]. Risk factors for the development of neurologic events include the development of hemolysis or thrombosis, or a VAD-specific infection, with up to 25% of patients with a neurologic event having a preceding infection [46].
Ethical considerations
As technology has improved, patients are now living to experience other diseases and morbidity. Part of the responsibility of caring forpatients supported by LVADs includes a thoughtful discussion of end of life planning and a device-living will. This need has been recognized more globally, with the Center for Medicare and Medicaid Services mandate that palliative care planning be part of the discussion with all DT implants.
Neurologic events associated with LVADs range from transientischemic attacks to cerebral embolic or hemorrhagic strokes. The incidences of these events have ranged from 6% to 9%, with lower incidences of ischemic stroke. There have been no differences noted in the incidence between DT and BTT candidates. Women appear to be at higher risk for all strokes [45,46]. Results from the 5th INTERMACS report reveal a 7% risk of stroke within the first two years after implantation [43]. Risk factors for the development of neurologic events include the development of hemolysis or thrombosis, or a VAD-specific infection, with up to 25% of patients with a neurologic event having a preceding infection [46].
Ethical considerations
As technology has improved, patients are now living to experience other diseases and morbidity. Part of the responsibility of caring forpatients supported by LVADs includes a thoughtful discussion of end of life planning and a device-living will. This need has been recognized more globally, with the Center for Medicare and Medicaid Services mandate that palliative care planning be part of the discussion with all DT implants.
Conclusions and Future Directions
Although research in the area of implantable ventricular assist devices has been ongoing since the 1960s, there has been a surge of development in the past decade following the commercialization of the technology. The increasing durability and reliability of the continuous flow pumps, coupled with the limited supply of donor hearts, have led to the acceptance of LVADs as standard of care for many patients with advanced heart failure. Although VAD-associated complications are quite prevalent with the current generation of devices, future advancements in the field hold the promise of minimizing future difficulties.The HeartWare MVAD pump is a continuous axial flow pump that is approximately one third the size of the current HVAD pump [47,48]. With continued miniaturization of the technology, future LVAD implantation may be achievable with minimally or less invasive surgery allowing for decreased perioperative morbidity and mortality. The small pump profile together with new cannula designs may allow for the avoidance of a full sternotomy and elimination of cardiopulmonary bypass through an apical approach [49]. The HeartMate III is an experimental centrifugal pump that is currently in the early investigational stages. The HeartMate III has several design elements aimed at reducing the thrombosis rate. The device’s textured surface provides a tissue to blood interface that is felt to be less thrombogenic than current models. Additionally, the HeartMate III incorporates pulsed technology that assists with pump wash out and intermittent aortic valve opening, with the goal to minimize thrombus formation and de novo aortic insufficiency [50]. These smaller devices may also find a role in the management of right ventricular failure and pulmonary artery hypertension. Concurrent with pump redesign, considerable research is underway in technologies that allow for fully implantable systems, thereby reducing or eliminating drivelineassociated infections. Transcutaneous energy transfer (TET) is a technology that allows for periodic and non-invasive recharging of an implanted battery. TET uses induction-heating which creates an electromagnetic current between coils located inside and outside of the body [51]. With continued advancements in design, device efficiency, reliability, durability and cost, LVADs will become even more readily available to patients with advanced heart failure.
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, et al. (2013) Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 127: e6-e245.
- Costanzo MR, Mills RM, Wynne J (2008) Characteristics of "Stage D" heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am Heart J 155: 339-347.
- Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, et al. (2013) The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant 32: 951-964.
- (2014) Organ Procurement and Transplantation Network.
- Stewart GC, Givertz MM (2012) Mechanical circulatory support for advanced heart failure: patients and technology in evolution. Circulation 125: 1304-1315.
- Liotta D, Hall CW, Henly WS, Cooley DA, Crawford ES, et al. (1963) Prolonged assisted circulation during and after cardiac or aortic surgery. Prolonged partial left ventricular bypass by means of intracorporeal circulation. Am J Cardiol 12: 399-405.
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, et al. (2001) Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 345: 1435-1443.
- Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, et al. (2007) Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 357: 885-896.
- Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, et al. (2009) Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361: 2241-2251.
- Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, et al. (2012) Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 125: 3191-3200.
- Pagani F, Rogers J (2014) A Prospective, Randomized, Controlled, Un-blinded, Multi-Center Clinical Trial to Evaluate the HeartWare® Ventricular Assist System (VAS) for Destination Therapy of Advanced Heart Failure (ENDURANCE). ClinicalTrials.gov Identifier: NCT01166347.
- Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, et al. (2014) Sixth INTERMACS annual report: A 10,000-patient database. J Heart Lung Transplant 33: 555-564.
- Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, et al. (2013) The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 32: 157-187.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: e240-e327.
- Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, et al. (2009) INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant 28: 535-541.
- Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, et al. (2011) Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant 30: 402-407.
- Baldwin JT, Mann DL (2010) NHLBI’s program for VAD therapy for moderately advanced heart failure: the REVIVE-IT pilot trial. J Card Fail 16: 855-858.
- Goda A, Williams P, Mancini D, Lund LH (2011) Selecting patients for heart transplantation: comparison of the Heart Failure Survival Score (HFSS) and the Seattle heart failure model (SHFM). J Heart Lung Transplant 30: 1236-1243.
- Levy WC, Mozaffarian D, Linker DT, Farrar DJ, Miller LW, et al. (2009) Can the Seattle heart failure model be used to risk-stratify heart failure patients for potential left ventricular assist device therapy? J Heart Lung Transplant 28: 231-236.
- Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, et al. (1991) Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83: 778-786.
- Stevenson LW, Miller LW, Desvigne-Nickens P, Ascheim DD, Parides MK, et al. (2004) Left ventricular assist device as destination for patients undergoing intravenous inotropic therapy: a subset analysis from REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure). Circulation 110: 975-981.
- Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, et al. (2014) Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 370: 33-40.
- Goldstein DJ, John R, Salerno C, Silvestry S, Moazami N, et al. (2013) Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 32: 667-670.
- Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, et al. (2012) Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol 60: 1764-1775.
- Bellumkonda L, Subrahmanyan L, Jacoby D, Bonde P (2014) Left ventricular assist device pump thrombosis: is there a role for glycoprotein IIb/IIIa inhibitors? ASAIO J 60: 134-136.
- Badiye A, Hernandez GA, Chaparro S (2014) Argatroban as novel therapy for suspected thrombosis in patients with continuous-flow left ventricle assist device and hemolysis. ASAIO J 60: 361-365.
- Thenappan T, Anderson AS, Jeevanadham V, Rich JD, Shah AP (2013) Treatment of left ventricular assist device thrombosis with extended catheter-directed intraventricular thrombolytic therapy. Circ Heart Fail 6: e27-e29.
- Stulak JM, Cowger J, Haft JW, Romano MA, Aaronson KD, et al. (2013) Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg 95: 1262-1267.
- Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, et al. (2011) Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 30: 115-123.
- John R, Lietz K, Schuster M, Naka Y, Rao V, et al. (2003) Immunologic sensitization in recipients of left ventricular assist devices. J Thorac Cardiovasc Surg 125: 578-591.
- Eckman PM, John R (2012) Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 125: 3038-3047.
- Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, et al. (2011) Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail 4: 779-784.
- Aggarwal A, Pant R, Kumar S, Sharma P, Gallagher C, et al. (2012) Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg 93: 1534-1540.
- Meineri M, Van Rensburg AE, Vegas A (2012) Right ventricular failure after LVAD implantation: prevention and treatment. Best Pract Res Clin Anaesthesiol 26: 217-229.
- de Potapov E, Meyer D, Swaminathan M, Ramsay M, El Banayosy A, et al. (2011) Inhaled nitric oxide after left ventricular assist device implantation: a prospective, randomized, double-blind, multicenter, placebo-controlled trial. J Heart Lung Transplant 30: 870-878.
- Takeda K, Naka Y, Yang JA, Uriel N, Colombo PC, et al. (2014) Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant 33: 141-148.
- Aissaoui N, Morshuis M, Schoenbrodt M, Hakim Meibodi K, Kizner L, et al. (2013) Temporary right ventricular mechanical circulatory support for the management of right ventricular failure in critically ill patients. J Thorac Cardiovasc Surg 146: 186-191.
- Morgan JA, John R, Lee BJ, Oz MC, Naka Y (2004) Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg 77: 859-863.
- Mudd JO, Cuda JD, Halushka M, Soderlund KA, Conte JV, et al. (2008) Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. J Heart Lung Transplant 27: 1269-1274.
- Jorde UP, Uriel N, Nahumi N, Bejar D, Gonzalez-Costello J, et al. (2014) Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circ Heart Fail 7: 310-319.
- Birati EY, Rame JE (2014) Left ventricular assist device management and complications. Crit Care Clin 30: 607-627.
- Hannan MM, Husain S, Mattner F, Danziger-Isakov L, Drew RJ, et al. (2011) Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 30: 375-384.
- Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, et al. (2013) Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 32: 141-156.
- Topkara VK, Kondareddy S, Malik F, Wang IW, Mann DL, et al. (2010) Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg 90: 1270-1277.
- Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, et al. (2014) Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 63: 880-888.
- Whitson BA, Eckman P, Kamdar F, Lacey A, Shumway SJ, et al. (2014) Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg 97: 2097-2103.
- McGee E Jr, Chorpenning K, Brown MC, Breznock E, Larose JA, et al. (2014) In vivo evaluation of the HeartWare MVAD Pump. J Heart Lung Transplant 33: 366-371.
- Slaughter MS, Sobieski MA 2nd, Tamez D, Horrell T, Graham J, et al. (2009) HeartWare miniature axial-flow ventricular assist device: design and initial feasibility test. Tex Heart Inst J 36: 12-16.
- Tamez D, LaRose JA, Shambaugh C, Chorpenning K, Soucy KG, et al. (2014) Early feasibility testing and engineering development of the transapical approach for the HeartWare MVAD ventricular assist system. ASAIO J 60: 170-177.
- Farrar DJ, Bourque K, Dague CP, Cotter CJ, Poirier VL (2007) Design features, developmental status, and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO J 53: 310-315.
- Rodriguez LE, Suarez EE, Loebe M, Bruckner BA (2013) Ventricular assist devices (VAD) therapy: new technology, new hope? Methodist Debakey Cardiovasc J 9: 32-37.