Journal of Cardiobiology
Download PDF
Case Report
Cutaneous Reaction to Continuously Inhaled Iloprost
Ramirez ML1*,Plescher B2, Santana-Acosta D2 and Khan DM3
1Pediatric Residency Program, Nicklaus Children’s Hospital, Miami, USA
2Division of Pediatric Critical Care, Nicklaus Children’s Hospital, Miami, USA
3Division of Cardiology, Nicklaus Children’s Hospital, Miami, USA
2Division of Pediatric Critical Care, Nicklaus Children’s Hospital, Miami, USA
3Division of Cardiology, Nicklaus Children’s Hospital, Miami, USA
*Address for Correspondence:Ramirez ML, Pediatric Residency Program, Nicklaus Children’s
Hospital 3100 SW 62nd Ave Miami, USA. E-mail Id: Monica.ramirezmendizabal@nicklaushealth.org
Submission:04 February, 2024
Accepted:06 March, 2024
Published:08 March, 2024
Accepted:06 March, 2024
Published:08 March, 2024
Copyright: ©2024 Ramirez ML, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Keywords:Pulmonary Vascular Resistance/Hypertension; Intensive
Care; Nitric Oxide; Pediatric; Congenital Heart Disease; Norwood
Procedure
Abstract
Pulmonary arterial hypertension is characterized by an increase
in pulmonary vascular resistance. It may occur in diverse clinical
settings, such as congenital heart disease, chronic lung disease,
connective tissue disease, or could be idiopathic. Pulmonary arterial
hypertension may cause significant morbidity and mortality. Iloprost
is a stable prostacyclin analog with vasodilatory properties. To
overcome its systemic side effects, the inhaled route has been used
to obtain pulmonary selectivity. We herein report an unusual case of a
cutaneous reaction to continuous inhaled iloprost. To our knowledge,
there are scarce case reports on cutaneous side-effects of inhaled
iloprost in the pediatric population. The objective of this clinical case
report is to highlight this unusual reaction to avoid incorrect diagnoses
and treatments.
Introduction
Inhaled iloprost is a prostacyclin analog utilized as a specific
pulmonary vasodilator in the treatment of pulmonary arterial
hypertension. Most reported adverse effects are due to its pulmonary
and systemic vasodilatory effect. In rare cases it can cause cutaneous
adverse effects.
Clinical Summary
A 2-year-old male with Trisomy 21, double outlet right
ventricle, unbalanced atrioventricular canal defect, aortic stenosis,
and hypoplastic aortic arch (single ventricle physiology), status
post Norwood and bidirectional Glenn procedure, was admitted
due to respiratory distress in the setting of an RSV infection. In a
previous cardiac catheterization, borderline high Glenn pressures
with a mean of 15-16 mmHg were noted. He was treated with noninvasive
ventilation, oxygen supplementation with FiO2 up to 100%,
and nitric oxide at 40 ppm. His oxygen saturation with supplemental
oxygen and nitric oxide ranged between 70-80%, expected with his
single ventricle physiology. Due to failure to improve, two weeks
after admission, he underwent cardiac catheterization in which his
left pulmonary artery was stented and a venovenous collateral was
occluded.
Twenty-two days into his hospital course, he deteriorated with
oxygen saturation dropping to 40-50%. Pulmonary vasodilator therapy
was further escalated by adding inhaled iloprost. To accomplish this,
20μg of iloprost was diluted in 20 mL of normal saline to create a 1
μg/mL solution, which was delivered via a non-invasive ventilation
using a RAM cannula at a rate equivalent to 1 μg/h. Over a period of
48 hours, the rate was gradually increased to 2 μg/h with a goal of 4
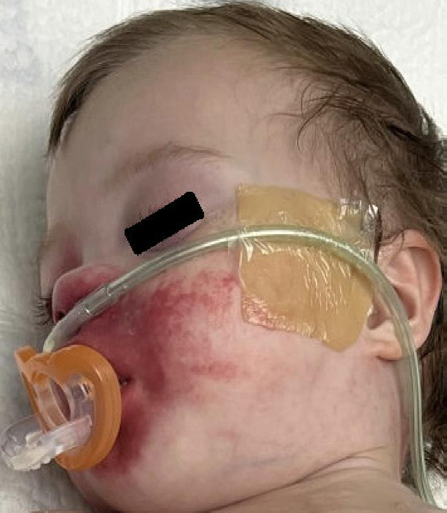
μg/h. When a rate of 2 μg/h was reached, an erythematous blanching
rash was noted around his nose. The rash was not raised, pruritic, or
did not cause the patient any discomfort. Several emollient creams
were attempted, including Vaseline and Aquaphor balm, without any
improvement. Due to concern that it could be a fungal rash, nystatin
cream was used, and intravenous fluconazole was given. Again, no
improvement was noted. As the rate increased to 3 μg/h and later to
4 μg/h, the rash spread around his nose to include his perioral region
[Figure 1]. In one instance, the patient removed his RAM cannula,
exposing his neck to the nebulized iloprost and the rash was noticed
transiently on his neck in the exposed area. Because of this, it was
assumed that the rash could be secondary to the vasodilatory effects of
the iloprost. Over the following days, as the iloprost was weaned and
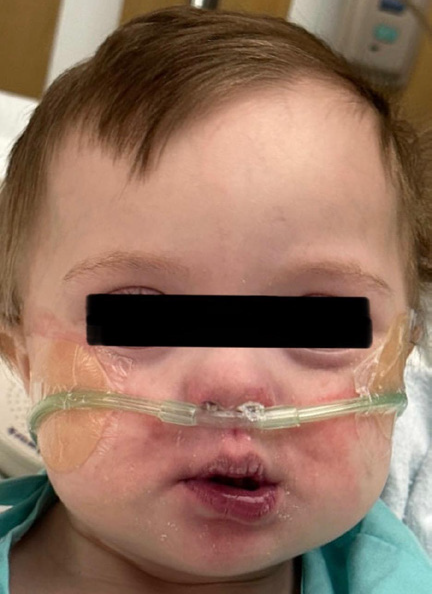
discontinued, the rash appeared to have a dose-dependent reaction.
The rash gradually self-resolved within 12 hours of discontinuing the
medication and significant improvement was seen within 24 hours
[Figure 2].
Discussion
Pulmonary vasoconstriction is a mechanism that is intrinsic to
the pulmonary vasculature. Smooth muscle of the pulmonary arteries
contain voltage-gated potassium and calcium channels sensitive to low
oxygen states. The vasoconstriction reflex is a contraction of vascular
smooth muscle in response to low regional partial pressure of oxygen.
It can help redirect blood flow away from poorly ventilated parts of the
lungs. Elevated resistance in the pulmonary circulation after chronic
hypoxia may lead to pulmonary hypertension. Pulmonary arterial
hypertension is characterized by a gradual increase in pulmonary
vascular resistance and can lead to progressive right ventricular
failure and death.[1]Nonetheless, increased awareness of the disease
and vasodilator therapy have improved patients’ survival and quality
of life. Pulmonary vasodilator treatment includes supplemental
oxygen, nitric oxide, endothelin receptor antagonist (bosentan and
ambrisentan), phosphodiesterase inhibitor (sildenafil), prostacyclin
analogues (epoprostenol, treprostinil, and iloprost), and guanylate
cyclase stimulators, to name a few. Although there is a beneficial
effect on the right ventricular afterload, utilizing vasodilators may
cause concomitant systemic vasodilation, ultimately aggravating the
patient’s condition. Chronic treatment with vasodilatory therapy
administered via intravenous or subcutaneous routes may be
limited by problems such as line infections, thrombosis, or site pain.
Administering vasodilator therapy via inhalation agents can help
target the lungs directly and prevent systemic vasodilation [2].
Inhaled iloprost is a prostacyclin analog that is selective for
pulmonary vasculature with an elimination half-life of 20–30
minutes.1Pharmacodynamic effects may be observed up to 30-90
minutes following a single inhaled dose. Prostacyclin is a potent
short-acting vasodilatory and platelet aggregation inhibitor produced
by the vascular endothelium. It can help decrease pulmonary vascular
resistance and increase cardiac output and systemic oxygen delivery.
Several reports have been published concerning inhaled iloprost in
acute pulmonary hypertensive crisis in children with pulmonary
arterial hypertension related to congenital cardiac disease. [3,4]
Olscheski et al. [5] reported that the local vasodilatory effect of inhaled
iloprost outlasts the disappearance of this agent from the systemic
circulation, this supported the hypothesis that drug deposition was
preferentially pulmonary as compared to systemic. An explanation
may be that some percentage of iloprost may be retained in the
perivascular lung tissue after nebulization. It may possess a longer
half-life than systemic iloprost as it is exposed to the catabolic
capacity of the liver.
Although inhaled iloprost therapy is currently approved for
treating pulmonary arterial hypertension in adults, little is known
about the potential side effects of inhaled iloprost in the pediatric
population. A study showed that a notable side effect of inhaled iloprost
was bronchoconstriction, potentially limiting its use. Nonetheless,
this side effect could be prevented with inhaled steroids before starting
treatment. The most common side effects reported were headache,
cough, and dizziness, which generally improved within several days
of initiation. [6] Feito Rodriguez et al. [7] reported a case in which
direct application of iloprost to the skin resulted in a sudden linear
erythematous facial rash that spontaneously resolved. It was noted that
on contact with the skin, iloprost invoked long-lasting but painless
erythema due to local vasodilatation. Additionally, a study reported a
cutaneous reaction to inhaled Treprostinil, a prostacyclin analog. The
mask-like erythematous reaction was believed to be a vasodilatory
effect secondary to Treprostinil. As our case, the erythema gradually
self-resolved over 48 hours after discontinuation of therapy [8].
Conclusion
Owing to our patient’s physiology and symptoms, we used
continuous inhaled iloprost therapy to improve ventilation-perfusion
mismatch. Our case depicts an unusual complication of inhaled
iloprost that may serve as a useful reference to other clinicians caring
for patients with pulmonary arterial hypertension and can help
prevent interruption of treatment due to the alarming appearance of
cutaneous adverse effects.
Disclosures and Freedom of Investigation:
There are no potential conflicts of interest with respect to the
research, authorship, and/or publication of this article. We did
not receive financial support for the research, authorship, and/or
publication of this article.Author’s Statement:
Consent for publication was granted by the patient’s mother
prior to submission.