Journal of Cardiobiology
Download PDF
Research Article
*Address for Correspondence: Thomas F. Lüscher, Director, Department of Cardiology, Cardiovascular Center, University Hospital Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland, Tel: +41 44 255 21 21; Fax: +41 44 255 44 01; E-mail: karlue@usz.uzh.ch
Citation: Siegrist PT, Enz T, Krasniqi N, Scherff F, Roth J, et al. Outcome of Percutaneous Patent Foramen Ovale Closure with the Premere Occluder — A Single Center Experience. J Cardiobiol. 2014;2(1): 6.
Copyright © 2014 Siegrist PT, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 2, Issue: 1
Submission: 10 November 2013 | Accepted: 14 December, 2013 | Published: 19 December, 2013
Reviewed & Approved by: Dr. MacArthur A Elayda, Department of Cardiovascular Epidemiology and Medical Informatics, Texas Heart Institute, USA
67% of the patients showed alterations of the carotid arteries on Doppler examination. Atrial fibrillation was confirmed only in 2% of the patients who were studied by Holter electrocardiogram.Baseline echocardiographic data are listed in Table 3. PFO in combination with atrial septal aneurysm was found in 28 patients (42%) while the common type of PFO (Figure 1) was documented in 27 patients (40%). The remaining 12 patients (18%) presented with a tubular PFO (Figure 2).
Immediate procedural successDevice implantation was successful in all 67 patients in the first interventional session. No periprocedural complications occurred, in particular any air embolism, device displacement or puncture site bleeding. Contrast injection confirmed proper device position in all patients immediately after placement. Residual shunt was observed in 2 patients (3%), however, the mild grade did not require implantation of a second closure device. Finally, chest X-ray on the following day also proved correct position of all closure devices. 67 patients (100%) could be discharged the day after the procedure.
Device thrombus formation occurred in one patient. The thrombus, measuring 10 x 10 mm, was attached to the device anchor in the right atrium as detected on follow-up TEE 22 days after PFO closure. The thrombus completely resolved under oral anticoagulation with phenprocoumon in addition to Aspirin without further complications, as confirmed echocardiographically on a second follow-up examination 30 days later.New onset atrial fibrillation was detected in one patient, 2 months after device implantation.One patient experienced a recurrent stroke 19 days after device closure of the PFO. Neuroimaging (MRI) displayed pontine infarction, however the etiology remained unclear. Notably no residual interatrial shunt was detected on echocardiography (TTE/TEE).TIA occurred in 1 patient, 18 months after implantation of occlusion device. Clinically the patient presented with transient ischemic attack (left sided hemiparesis) with complete remission after 9 hours. Repeated echocardiography revealed malposition of the occlusion device with mild residual right-to-left shunt. The patient subsequently underwent surgical device extraction and patch closure of the interatrial defect. No further complications were reported hereafter.Significant residual interatrial shunt (> 20 microbubbles) was detected in 6 patients (9.4%). In 3 patients (4.7%) follow-up echocardiography revealed a newly developed minimal aortic regurgitation. In 2 patients (2.9%) a new minimal tricuspid regurgitation was detected and in 1 patient (1.9%) an increase from minimal to mild tricuspid regurgitation was noted. Table 5 shows valve function at baseline and follow-up.
Of 10 Patients with migraine prior to PFO closure 70% were free of migraine on follow-up examination. However, 2 patients reported new onset migraine after PFO occlusion.
Outcome of Percutaneous Patent Foramen Ovale Closure with the Premere Occluder — A Single Center Experience
Patrick T. Siegrist, Tim Enz, Nazmi Krasniqi, Frank Scherff, Janice Roth, Felix Tanner, Roberto Corti and Thomas F. Lüscher*
- Department of Cardiology, Cardiovascular Center, University Hospital Zurich, Switzerland
*Address for Correspondence: Thomas F. Lüscher, Director, Department of Cardiology, Cardiovascular Center, University Hospital Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland, Tel: +41 44 255 21 21; Fax: +41 44 255 44 01; E-mail: karlue@usz.uzh.ch
Citation: Siegrist PT, Enz T, Krasniqi N, Scherff F, Roth J, et al. Outcome of Percutaneous Patent Foramen Ovale Closure with the Premere Occluder — A Single Center Experience. J Cardiobiol. 2014;2(1): 6.
Copyright © 2014 Siegrist PT, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 2, Issue: 1
Submission: 10 November 2013 | Accepted: 14 December, 2013 | Published: 19 December, 2013
Reviewed & Approved by: Dr. MacArthur A Elayda, Department of Cardiovascular Epidemiology and Medical Informatics, Texas Heart Institute, USA
Abstract
Introduction: Percutaneous closure of patent foramen ovale (PFO) has become a widely used procedure in patients with suspected paradoxical embolism. Despite being generally safe and effective, the minimally invasive intervention has been associated with several serious complications such as air embolism, cardiac perforation, device embolization, device thrombus formation and puncture site problems. Unlike the conventionally used devices originally designed for closure of atrial septum defects, the novel Premere PFO Closure System (St. Jude Medical, St. Paul, MN, USA) was exclusively designed for PFO occlusion. We therefore aimed to evaluate the safety and efficacy of the novel Premere PFO Closure System in patients with PFO and a previous transient ischemic attack (TIA) or stroke.Methods: 67 patients (mean age 47 ± 29 years) who underwent PFO closure with the novel Premere device were analyzed retrospectively. Indications for PFO closure were prior stroke (n=42), transient ischemic attack (n=20), or other related clinical events such as peripheral embolism (n=5). PFO was documented in all patients by transthoracic and transesophageal echocardiography. Procedural success was confirmed by fluoroscopy with contrast injection through the guiding catheter. Mid-term safety and efficacy was evaluated by clinical follow-up, including echocardiography (TTE/TEE) scheduled at 6 months after PFO closure.
Results: Follow-up data was available in 64 patients (95.5%). Device implantation was successful in all patients without any periprocedural complications. At clinical follow-up after a median of 189 ± 135 days, one patient (1.6%) presented with device thrombus, which resolved under oral anticoagulation without further complications. Another was diagnosed with new onset of atrial fibrillation. One patient each had experienced a recurrent stroke and a transient ischemic attack. While in the former case no residual shunt could be detected, the later was likely related to device malposition. At echocardiographic follow-up, significant residual shunt was noted in 6 patients (9.4%).Conclusion: In our series we demonstrate that percutaneous PFO closure using the dedicated Premere occluder is safe and effective. Type and rate of complications are in line with other studies assessing this closure device.
Background
A patent foramen ovale (PFO) of the interatrial septum occurs in more than 25% of the general population [1], potentially promoting cryptogenic stroke [2-4]. The underlying pathophysiologic mechanism is believed to be paradoxical embolism with distinct reasons for right-to-left shunting. Elevation of right ventricular pressure, transient spontaneous reversal of the left to right atrial pressure differential with each cardiac cycle, persistent elevation of right above left atrial pressure induced by respiratory maneuver and aberrant flow redirection across the PFO due to a right atrial mass have been described as possible etiologies [5,6].In 1992 Bridges et al. introduced percutaneous device closure of PFO to eliminate the pathway of paradoxical embolism [7]. Indeed, there is accumulating evidence that transcatheter closure of PFO prevents recurrent embolic events in selected patients [8-10] and appears to be at least as effective as medical therapy [8,11,12]. Providing a far less invasive intervention than conventional open chest surgery, transcatheter device closure of PFO became a widely used procedure.Despite being a generally safe, minimally invasive intervention, several complications such as cardiac perforation, air and device embolization, supraventricular arrhythmias, thrombus formation on the device and puncture site problems have been reported [13]. Moreover is a bulky occlusion device likely to alter cardiac chamber structure and consequently to induce or worsen valvular regurgitation [14].Unlike the conventionally used devices that were originally designed for closure of atrial septum defects, the novel Premere PFO Closure System (St. Jude Medical, St. Paul, MN, USA) was exclusively designed for PFO closure. Therefore specific anatomical and functional characteristics could be addressed, aiming to further decrease complication rate. The small surface area and low profile of the left anchor arm not only allows rapid and complete endothelialization but also reduces the potential of thrombus formation and tissue erosion. The two nitinol anchors are connected with an adjustable tether designed to adapt for various PFO tunnel lengths with minimal septal distortion [15].Our study aimed to evaluate the safety and efficacy of the novel Premere PFO Closure System in patients with a previous cryptogenic stroke or transient ischemic attack (TIA), and echocardiographic diagnosis of PFO.Methods
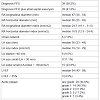
Patient populationWe retrospectively analyzed 67 patients (41 male, mean age 49.2 ± 29 years) who underwent PFO closure with the 25 mm Premere PFO closure system between March 2008 and December 2012 at our institution. Indications for PFO closure were prior cryptogenic stroke, TIA, or peripheral arterial embolism believed to be related to paradoxical embolism. PFO was documented in all patients by transthoracic and transesophageal echocardiography with color Doppler and microbubble contrast imaging at rest or during Valsalva maneuver. Additional baseline evaluation included neuroimaging, Holter electrocardiograms, sonographic/Doppler evaluation of the carotid and vertebral arteries, workup for current thromboembolic disease, laboratory evaluation of thrombophilia and coronary angiography. Data were retrieved from the electronic patient charts at our institution as well as from follow-up reports of associated hospitals and ambulatory clinics. In single cases lacking followup data were complemented by telephonic patient interviews. The present study was approved by the Cantonal Ethics Committee of Zurich, Switzerland.
Device and procedure
Prior to implantation written informed consent was obtained from all patients. The procedure was performed without general anesthesia via femoral venous access. Under fluoroscopic guidance a guidewire was advanced through the PFO into a pulmonary vein, followed by balloon sizing of the PFO diameter. Premere device selection was made at the operator’s discretion. Contraindications for selecting the Premere occluder were anatomical reasons such as additional atrial septum defect or multiple septal defects and Chiari network.The Premere PFO closure system consists of two low profile anchors connected with a length adjustable tether, specifically designed for percutaneous, transcatheter PFO closure. Through a 9-14 Fr delivery sheath, the left-sided anchor was deployed in the left atrium and retracted by the polyester tether until completely attached to the interatrial septum. Subsequently the delivery sheath was removed into the right atrium followed by deployment of the right-sided anchor. After fluoroscopic confirmation of satisfactory occluder position, the device was released by cutting the tether with the catheter. Immediately after the implantation, procedural success was confirmed by fluoroscopy with contrast injection through the guiding catheter. Before discharge of the patient a chest x-ray was performed to exclude device dislocation.
Follow-up
Follow-up examinations were either performed at our institution or with the referring hospital/cardiologist. The recommended follow-up regimen included patient history, physical examination and transthoracic or transesophageal echocardiography using microbubble contrast imaging six months after PFO closure.Postintervention medical treatment consisted of aspirin 100 mg per day for 6 months (if no indication for unlimited therapy) and Clopidogrel 75 mg per day for 1 month. Endocarditis prophylaxis according to current guidelines was recommended for the period of 6 months.Follow-up data were analyzed regarding safety and efficacy of the Premere PFO closure system. Safety aspects are expressed by the incidence of adverse events, including procedural and procedure related complications such as atrial fibrillation and thrombus formation. Additionally, the effect of the Premere occluder on heart valve regurgitation was analyzed. Efficacy was judged by the rates of complete closure and recurrent thromboembolic events.
Statistical analysis
Data are reported as means ± standard deviation (SD) or as means and range for continuous variables and as numbers of patients and percentages for categorical variables.
Results
Patients and baseline examinationsPatient characteristics and results of baseline examinations are shown in Table 1 and indications for PFO closure are presented in Table 2. In all patients a thromboembolic event was documented and attributed to paradoxical embolism according to baseline examinations. In all but 5 patients embolism resulted in ischemic stroke or transient ischemic attack. More than a quarter of the study population sustained at least one recurrent event prior to the intervention and the median time interval from the last event to PFO closure was 73 days.
Findings at follow-up
Follow-up data was available in 64 patients (95.5%). Three patients (4.5%) were lost to follow-up due to clinical follow up in external clinics. At clinical follow-up after a median of 189 days (range: 54 - 420 days) we found an adverse event rate of 6.3% (4/64 patients) and a complete closure rate of 90.6% (58/64 patients). Follow-up data are listed in Table 4.
Discussion
In the present study we demonstrate that PFO closure using the novel Premere PFO Closure System is safe and effective. Our findings are in line with other large series of percutaneous PFO closure using the Premere device [15-18].Indeed, no complications occurred during device implantation. Being specifically designed for PFO closure, the Premere system features several innovations that allow a safe and effective implantation procedure. The flexible, independent anchors offer excellent control during placement, an innovative tether design provides direct tactile feedback of the septum, the device is easy to reposition or to retrieve if necessary and the novel introducer sheath prevents air embolism. Consistently, other studies also report a very low immediate interventional event rate. While Buscheck [15] and Rigatelli [18] did not experience any procedural complications likewise, the larger series of Stanczak [17] found an intraprocedural adverse event rate of 1.1% (consisting of two acute coronary syndromes and one device dislocation). Earlier studies involving various occlusion devices reported a major complication rate of 1.5% [19] and 2.5% [20], including death, major hemorrhage, need for surgical intervention, cardiac tamponade and device or air embolization.
After a median follow-up time of 189 days, the clinical event rate was still low, but not negligible. In fact, a recurrent stroke, TIA, device thrombus and atrial fibrillation were noted in one patient each. These complications are known after PFO closure.
Recurrent stroke and TIA have been detected in numerous studies and appear to occur less frequent with newer devices [21]. Among the studies using the Premere occluder exclusively, neither Buscheck nor Rigatelli reported recurrent thromboembolic events during the follow-up period of 6 months and 40 ± 10.9 months, respectively. Stanczak however found a recurrent stroke or TIA in 3.5% of the 260 patients during follow-up of 19.3 ± 14.2 months. Yet in none of those cases a definite stroke mechanism could be identified. Thus, in the absence of a residual interatrial shunt or a thrombus formation on the left atrial anchor of the occluder, the stroke mechanism might be unrelated to the occlusion device or even the PFO. Indeed, in the patient with recurrent stroke in our study, no such etiology could be detected. Conversely, in the patient presenting with a recurrent TIA, dislocation of the occlusion device was found.
Device thrombus is another infrequent but potentially serious complication of PFO closure. The exceptionally small surface area of the left-sided anchor of the Premere occluder aims to reduce the incidence of thrombus formation. We found thrombus formation in one patient. Notably the thrombus was located on the right atrial side and resolved completely under treatment with aspirin and oral anticoagulation. Consistently, the Premere occluder was associated with a very low rate of thrombus formation of 0 – 0.4% among the studies of Buscheck, Rigatelli and Stanczak. By contrast, earlier and bulkier devices were associated with a higher incidence of thrombus formation (up to 7%) [21,22].
New onset of atrial fibrillation is a complication seen more frequently after device closure of PFO. While we report only one case of new onset of atrial fibrillation (1.6%) at follow-up after 6 months, Buscheck and Stanczak found an incidence of 1.5% (at follow-up after 6 months) and 3.1% (mean follow-up 19.3 months), respectively. With other PFO occlusion devices atrial fibrillation has been noted in 0.9 – 7% of cases after implantation [23-25]. Since atrial tachyarrhythmias seem to occur more frequently in patients who received larger occlusion devices, irritation and wound healing process in the left atrium appears to be a possible underlying mechanism [26]. However, direct association of PFO device closure with new onset of atrial fibrillation has been questioned by a recently published meta-analysis, reporting that PFO closure might even be associated with reduction in the prevalence of atrial fibrillation [27]. In fact, direct association with atrial fibrillation has only been found for large PFO (≥50 microbubbles), which appeared to be a significant predictor of occurrence of atrial fibrillation [28]. Further prospective randomized studies are needed to clarify this important issue, particularly with regard to the fact that some recurrent or even index strokes/embolisms might finally be associated with paroxysmal atrial fibrillation, leaving the PFO as an innocent companion.
Acute closure rate of the present study as assessed angiographically immediately after device placement was excellent (96.6%). However, at echocardiographic follow-up (TTE/TEE) residual shunt was documented more frequently in our study as compared to other studies evaluating the Premere occluder. Among the 34.3% residual shunts detected, 9.4% were considered significant (>20 microbubbles). While Stanczek reports residual shunts in 7.3% without specification of severity, Bruscheck found 14%, the majority being small (<10 microbubbles). The substantially longer follow-up time of Stanczek’s report (19.3 ± 14.2 month) compared to our study (189 days) is likely to have contributed to a higher closer rate, since residual shunts continue to disappear even after 6 months by endothelialization of the device [29]. In addition to the low profile of the left anchor, allowing rapid and complete endothelialization, the Premere occluder features a length adjustable tether between the two independently pivoting anchors, aiming to improve closure rate especially for long PFO tunnels. Indeed, Rigatelli found a closure rate of 98.5% in selected patients with long PFO tunnel or hypertrophic rims and absence of moderate/severe atrial septum aneurysm (ASA). In a study using four different PFO occluding devices (including the Premere occluder), extend of ASA (per mm) has been found an independent predictor of residual shunt [30]. Thus, the high percentage of ASA (42%) in our cohort may have contributed to the higher rate of residual shunts. There are studies with other PFO-occluders such as Amplatzer or Gore Helex with a high percentage of atrial septal aneurysm. In these studies was the rate of residual shunts higher than in patients without ASA. The rate of residual shunts was dependent on design of PFO-occluder. For example Amplatzer PFO-Occluder had a lower rate of residual shunts as Gore Helex PFO-Occluder [31]. Knowing these facts it is very important to know the anatomy of the PFO in each patient, to be able to decide which occluder is the optimal one. In patients with tubular PFO without ASA seems Premere to be the right one [18]. Selecting occlusion devices according to the different anatomical characteristics of a PFO and the interatrial septum appears favorable to further enhance closure rate; however, further specific studies are needed to elucidate the strengths and weaknesses of different PFO occluders with respect to individual anatomical features.
Our study shows the real life results of PFO-Occlusion with a special device.
It shows that a carefully selection of patients for PFO-closure is very important. The anatomy of the PFO is in this case one of the most important selection criteria, for example patients with ASA (atrial septum aneurysm) should not be treated with flexible PFO-occluders such as Premere, because of the higher rate of residual shunts during the follow up period. Patients with tubular PFO who are treated with Premere-occluder have the best results, because in these patients the closure of PFO is safe (low rate of thrombus formation on the left anchor) and there is a low rate of residual shunt.
Recent echocardiographic follow-up studies indicated that newly induced or worsened valve regurgitations might pose another safety aspect [14,32]. The underlying alteration of cardiac chamber structure is likely to occur less with the slender and flexible Premere occluder than with more bulky devices. Indeed no significant impact on valve function was detected in the present study.
Limitations
The present study has all the limitations inherent to a retrospective single-center study. Since selection of the Premere occluder for PFO closure was not done according to any previously specified criteria a potential patient selection bias cannot be ruled out. Furthermore, the mean follow-up period of 189 days is still short, considering the low incidence of adverse events. However, with the exception of effects on cardiac chamber structure, most directly device related complications are likely to occur within the first month until endothelialization has completed. Finally, we did not directly compare the Premere occluder to any other PFO occlusion device in a randomized trial. Thus, final conclusions regarding superiority to other devices cannot be drawn.Conclusions
Our data confirm that percutaneous PFO closure using the novel Premere PFO Closure System is safe and effective. The slender and flexible design appears to reduce potential complications such as thrombus formation on the left anchor, erosion and alteration of chamber structure. Performance may be further enhanced by device selection according to individual anatomical characteristics. Further studies are needed to elaborate respective criteria.Acknowledgements
Dr. Patrick T. Siegrist and Dr. Tim Enz has contributed equally to this work.References
- Hagen PT, Scholz DG, Edwards WD (1984) Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 59: 17-20.
- Overell JR, Bone I, Lees KR (2000) Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 55: 1172-1179.
- Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A (2007) Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 357: 2262-2268.
- Alsheikh-Ali AA, Thaler DE, Kent DM (2009) Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 40: 2349-2355.
- Lehmeyer S, Lindhoff-Last E (2011) New aspects of paradoxical embolism. Vasa 40: 31-40.
- Langholz D, Louie EK, Konstadt SN, Rao TL, Scanlon PJ (1991) Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension. J Am Coll Cardiol 18: 1112-1117.
- Bridges ND, Hellenbrand W, Latson L, Filiano J, Newburger JW, et al. (1992) Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 86: 1902-1908.
- Schuchlenz HW, Weihs W, Berghold A, Lechner A, Schmidt R (2005) Secondary prevention after cryptogenic cerebrovascular events in patients with patent foramen ovale. Int J Cardiol 101: 77-82.
- Wahl A, Juni P, Mono ML, Kalesan B, Praz F, et al. (2012) Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation 125: 803-812.
- Windecker S, Wahl A, Nedeltchev K, Arnold M, Schwerzmann M, et al. (2004) Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 44: 750-758.
- Cifarelli A, Musto C, Parma A, Pandolfi C, Pucci E, et al. (2010) Long-term outcome of transcatheter patent foramen ovale closure in patients with paradoxical embolism. Int J Cardiol 141: 304-310.
- Wohrle J (2006) Closure of patent foramen ovale after cryptogenic stroke. Lancet 368: 350-352.
- Meier B (2009) Catheter-based closure of the patent foramen ovale. Circulation 120: 1837-1841.
- Krasniqi N, Roth J, Siegrist PT, Toggweiler S, Gruner C, et al. (2012) Percutaneous closure of patent foramen ovale and valvular function -- effect of the amplatzer occluder. J Invasive Cardiol 24: 274-277.
- Buscheck F, Sievert H, Kleber F, Tiefenbacher C, Krumsdorf U, et al. (2006) Patent foramen ovale using the Premere device: the results of the CLOSEUP trial. J Interv Cardiol 19: 328-333.
- Kleber FX, Winkelmann A, Stretz A, Sonntag SM, Bruch L, et al. (2010) Occlusion of PFO with a dedicated adjustable device: influence on one year outcome. EuroIntervention 6: 367-370.
- Stanczak LJ, Bertog SC, Wunderlich N, Franke J, Sievert H (2012) PFO closure with the Premere PFO closure device: acute results and follow-up of 263 patients. EuroIntervention 8: 345-351.
- Rigatelli G, Cardaioli P, Dell’avvocata F, Giordan M, Chinaglia M (2011) Premere occlusion system for transcatheter patent foramen ovale closure: mid-term results of a single-center registry. Catheter Cardiovasc Interv 77: 564-569.
- Khairy P, O’Donnell CP, Landzberg MJ (2003) Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 139: 753-760.
- Wahl A, Kunz M, Moschovitis A, Nageh T, Schwerzmann M, et al. (2008) Long-term results after fluoroscopy-guided closure of patent foramen ovale for secondary prevention of paradoxical embolism. Heart 94: 336-341.
- Staubach S, Bertog S, Wunderlich N, Sievert H (2009) Late complications of transcatheter closure of patent foramen ovale: new technology and new outcomes. Future Cardiol 5: 503-510.
- Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, et al. (2004) Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol 43: 302-309.
- Spies C, Khandelwal A, Timmermanns I, Schrader R (2008) Incidence of atrial fibrillation following transcatheter closure of atrial septal defects in adults. Am J Cardiol 102: 902-906.
- Staubach S, Steinberg DH, Zimmermann W, Wawra N, Wilson N, et al. (2009) New onset atrial fibrillation after patent foramen ovale closure. Catheter Cardiovasc Interv 74: 889-895.
- Taaffe M, Fischer E, Baranowski A, Majunke N, Heinisch C, et al. (2008) Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder). Am J Cardiol 101: 1353-1358.
- Alaeddini J, Feghali G, Jenkins S, Ramee S, White C, et al. (2006) Frequency of atrial tachyarrhythmias following transcatheter closure of patent foramen ovale. J Invasive Cardiol 18: 365-368.
- Jarral OA, Saso S, Vecht JA, Harling L, Rao C, et al. (2011) Does patent foramen ovale closure have an anti-arrhythmic effect? A meta-analysis. Int J Cardiol 153: 4-9.
- Bonvini RF, Sztajzel R, Dorsaz PA, Righini M, Bonvin C, et al. (2010) Incidence of atrial fibrillation after percutaneous closure of patent foramen ovale and small atrial septal defects in patients presenting with cryptogenic stroke. Int J Stroke 5: 4-9.
- Yared K, Baggish AL, Solis J, Durst R, Passeri JJ, et al. (2009) Echocardiographic assessment of percutaneous patent foramen ovale and atrial septal defect closure complications. Circ Cardiovasc Imaging 2: 141-149.
- Hammerstingl C, Bauriedel B, Stusser C, Momcilovic D, Tuleta I, et al. (2011) Risk and fate of residual interatrial shunting after transcatheter closure of patent foramen ovale: a long term follow up study. Eur J Med Res 16: 13-19.
- Musto C, Cifarelli A, Fiorilli R, De Felice F, Parma A, et al. (2012) Gore Helex septal occluder for percutaneous closure of patent foramen ovale associated with atrial septal aneurysm: short- and mid-term clinical and echocardiographic outcomes. J Invasive Cardiol 24: 510-514.
- Schoen SP, Boscheri A, Lange SA, Braun MU, Fuhrmann J, et al. (2008) Incidence of aortic valve regurgitation and outcome after percutaneous closure of atrial septal defects and patent foramen ovale. Heart 94: 844-847.