Journal of Cardiobiology
Download PDF
Research Article
*Address for Correspondence: Sandro Sponga, Cardiothoracic Department, University Hospital of Udine, P.le S.M. della Misericordia, 15, 33100 Udine, Italy, Tel: +39 0432 552430/31; Fax +39 0432 552975; E-mail: sandro_sponga@yahoo.com
Citation: Sponga S, Della Mattia A, Mazzaro E, Guzzi G, Daffarra C, et al. Treatment of Tricuspid Regurgitation Following Heart Transplantation: Surgical Approach and Results. J Cardiobiol. 2013;1(1): 4.
Copyright © 2013 Sponga S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 1, Issue: 1
Submission: 26 August 2013 | Accepted: 27 September 2013 | Published: 01 October 2013
Reviewed & Approved by: Li Jianhua, Department of Pediatric Cardiothoracic Surgery, Children’s Hospital Zhejiang University School of Medicine, China
Treatment of Tricuspid Regurgitation Following Heart Transplantation: Surgical Approach and Result
Sponga S*, Della Mattia A, Mazzaro E, Guzzi G, Daffarra C, Pavoni D, Spagna E, Tursi V and Livi U
- Cardiothoracic Department, University Hospital of Udine, Italy
*Address for Correspondence: Sandro Sponga, Cardiothoracic Department, University Hospital of Udine, P.le S.M. della Misericordia, 15, 33100 Udine, Italy, Tel: +39 0432 552430/31; Fax +39 0432 552975; E-mail: sandro_sponga@yahoo.com
Citation: Sponga S, Della Mattia A, Mazzaro E, Guzzi G, Daffarra C, et al. Treatment of Tricuspid Regurgitation Following Heart Transplantation: Surgical Approach and Results. J Cardiobiol. 2013;1(1): 4.
Copyright © 2013 Sponga S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Cardiobiology | ISSN: 2332-3671 | Volume: 1, Issue: 1
Submission: 26 August 2013 | Accepted: 27 September 2013 | Published: 01 October 2013
Reviewed & Approved by: Li Jianhua, Department of Pediatric Cardiothoracic Surgery, Children’s Hospital Zhejiang University School of Medicine, China
Abstract
Background: Tricuspid valve regurgitation is common after heart transplantation, but the need for replacement or repair is rare. Patients with signs of advanced right-sided heart failure are considered at higher risk for surgery. The aim of this study is to evaluate our experience in the treatment of tricuspid valve regurgitation following heart transplantation.Methods: We reviewed our overall experience in heart transplantation between 1985-2013 (508 cardiac transplants). Five patients (1%) underwent surgical treatment for severe tricuspid regurgitation with right ventricular failure at a median interval of 81 (8-138) months after transplantation. The mechanism of TR was leaflet prolapse, due to chordal rupture after biopsy injury combined with annular dilatation in 3 patients, leaflets thetering combined with annular dilatation in 2. Surgical approach complied in right minithoracotomy and peripheral vessels cannulation for extra corporeal circulation, the procedure was carried out on beating heart.
Results: Three patients underwent bioprosthesis valve replacement and 2 ring implantation. Two patients died as consequence of refractory right ventricular failure despite temporary mechanical circulatory support. One patient developed renal failure and cerebral ischemia and another one needed prolonged ventilatory support. During the follow-up (27±13 months) the 3 survivors improved their functional NHYA class (< II in all), neither recurrence of right ventricular failure nor prosthetic or repair failures occurred.
Conclusions: The treatment of severe tricuspid regurgitation in cardiac transplant patients is associated with early high morbidity and mortality but with satisfactory mid term results. The presence of severe right ventricular dysfunction seems to have a significant prognostic impact on patients’ outcome.
Keywords
Tricuspid regurgitation; Heart transplantationIntroduction
Tricuspid regurgitation (TR) is a common finding after orthotopic heart transplantation (OHT) and it is considered a frequent valve pathology at early and long term follow up with a reported rate ranging from 19% to 84% [1,2]. Etiology of TR has been reported as a consequence of endomyocardial biopsy (EMB) by tricuspid valve leaflets or chordal damage [3,4]. TR is generally asymptomatic and the diagnosis is made by echocardiography [5]. TR rarely evolves to symptomatic disease and it is usually well controlled with medical therapy, otherwise surgical treatment may be required in case of persistent right ventricular dysfunction [1,6].The purpose of this study is to evaluate our experience in tricuspid valve surgery following OHT.
Patients and Methods
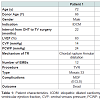
Between 1985 and 2013, 508 OHT (in 502 patients) were performed at Udine University Hospital. In this report, we included five patients (mean age was 61.8±11.7 years, all males) who underwent tricuspid valve surgery for symptomatic severe TR with right ventricular dysfunction (NYHA class III-IV). Indications and surgical technique are reported in Table 1.
In our institution following OHT every patient received clinical evaluation, echocardiography, EMB and coronary angiography.
Echocardiograhpy included grading of tricuspid regurgitation, evaluation of its pathogenetic mechanism as leaflet flail or tethering and annular dilatation and assessment of right ventricular function (ventricular dimensions, mean right ventricular fractional area and tricuspid annular plane systolic excursion).
EMBs were performed weekly during the first month after heart transplantation, every fifteen days until the third month and monthly through the first year. Rejection was diagnosed according to the last International Society of Heart and Lung Transplantation criteria [7]. Routine immunosuppressive protocol included cyclosporine, mycophenolate, prednisone and anti-thymocyte globulin induction. The presence of coronary vasculopathy was routinely investigated with standard coronary angiography every 2 years or every year combined with intravascular ultrasound (IVUS) to evaluate the progression of identified stenosis.
Surgical techniqueMinimal invasive approach was adopted in all cases. Patients were positioned supine with the right side of the chest slightly elevated. In all cases a double-lumen endotracheal tube and a transesophageal echocardiography (TEE) were employed. A mini right anterolateral thoracotomy was performed through the fourth intercostal space more anterior than usually made for mitral valve approach. Cannulation of the femoral artery and both femoral and jugular veins were used. After systemic heparinization, patients were placed on cardiopulmonary bypass to allow a beating heart operation. The right atrium was then opened and the tricuspid valve was exposed using an atrial retractor. The tricuspid valve was explored to check any leaflet prolapse, chordal rupture and annular dilatation (Figures 1 and 2). The tricuspid valve repair (TVRP) or replacement (TVR) was performed under both direct vision and thoracoscopy. Temporary pace wires have been placed on right ventricle before CPB weaning. At the end of surgery TEE was performed to evaluate the results of valve repair or replacement and ventricular function. A drainage tube was placed in the right pleural chest and the tip of a second small drain was introduced in the pericardial space. The thoracotomy was closed in a standard fashion.
All statistical analyses were performed using the SPSS software package (version 12.0; SPSS, Chicago, IL, USA). Continuous data are reported by giving the mean ± standard deviation or median and the range of values observed Incidence rates of events are reported by giving the number of patients experiencing the event followed by the corresponding percentage.
Results
The median interval for the development of TR after OHT was 81 (8-138) months after transplantation. No donors had showed TR before heart harvesting, mean donor age was 52±11 years, one was 66 year old.Among the 5 patients, one needed PTCA and stenting of descending anterior, circumflex and right coronary artery at 8 years. The mean number of EBM performed was 13±3. The mean time from the last EMB to tricuspid surgery was 51±46 months. At the echocardiography, the pathogenetic mechanism of TR was leaflet flail in 3 patients, due to chordal rupture combined with annular dilatation. In one case, EMB was quite close (5 months) to the detection of valvular damage and leaflet flail. Tricuspid regurgitation was due to leaflet tethering combined with annular dilatation in 2 cases (Table 1). Three patients (60%) had preserved left ventricular ejection fraction (LVEF), and 2 patients (40%) had mild left ventricular dysfunction [mean LVEF calculated was 57.2±8.1%]. All patients showed right ventricular dysfunction with dilated chambers (TDV 169±10 ml, TSV 74±30 ml) the mean right ventricular fractional area change observed was 31±8% and mean TAPSE was 13±5 mm.
The preoperative central venous pressure, mean pulmonary arterial pressure, pulmonary capillary wedge pressure, pulmonary arterial resistance and mean cardiac index were 17±3.74 mmHg, 25.4±3.57 mmHg, 16.6±3.84 mmHg 2.12±0.83 UW and 2.17±0.22 ml/m2, respectively.
Among all patients, 3 (60%) had tricuspid valve replacement (TVR) with a bioprosthesis (Mosaic 33, Hancock and Hancock II 31 Medtronic, Minneapolis, MN, USA) and the remaining 2 (40%) had tricuspid valve repair (TVRP) with a tricuspid ring (Edwards MC 30 and Edwards MC 32, Edwards Lifesciences, Irvine, CA, USA) (Table 1). At post-operative TEE all patients demonstrated a TR < mild in all cases. Central venous pressure decreased from 20±8 to 14±8 mmHg at the end of surgery.
Two patients died postoperatively due to multi-organ failure as a consequence of irreversible and severe right ventricular dysfunction at 5 and 20 days. Both patients were treated with mechanical circulatory support at the end of surgery because of impossible weaning from CPB despite maximal inotropic support.
Among commorbidities, 1 patient developed renal failure and a transient ischemic attack. Prolonged ventilatory support (4 days) was necessary in another patient. Mean troponin I levels after cardiac surgery was 7.4±2.4 ng/mL. Mean ICU and in Hospital stay was 8.2±6.6 and 11.8±7.1 days respectively.
During the follow-up (27±13 months), the 3 survivors improved their functional NHYA class (NYHA was < II in all), no patient had recurrence of right ventricular failure with a minimal dose of diuretics (the furosemide dose ranged from 25 to 75 mg daily) and no prosthetic or repair failures was recorded. One patient 12 months after surgery developed an endocarditis by Streptococcus gallolyticus treated successfully with medical therapy. The last echocardiography control revealed a mild TR in 2 patients and trivial in the other survivor, without significant right ventricular dysfunction in all. The maximum and mean tricuspid prosthetic gradients observed were 8.3±4.0 mmHg and 4.7±2.5 mmHg, respectively.
Discussion
Tricuspid valve surgery has been reported after OHT with a rate of 1-6% and a mean time since OHT of 12 to 21 months [1,2,4,9]. The most common cause of TR is EMB by damage of tricuspid valve leaflets or chordae [3]. Among other factors reported to have an impact on TR there are surgical technique for OHT, acute and chronic rejection, pulmonary hypertension and ischemic time [3].In 3 of our patients the mechanism of TR was leaflet prolapse, due to chordal rupture combined with annular dilatation even if in only one case EMB had been performed closely to echocardiographic diagnosis. In the remaining patients, vice versa, TR was due to leaflet tethering and annular dilatation. In these cases, myocardial ischemia could have been determinant: in one case the donor was 66 years old and in the other one multiple PTCA were performed for coronary allograft vasculopathy.
Endomyocardial biopsy is the “gold standard” of rejection surveillance after cardiac transplantation. However, it is invasive, and recognized to be cause of complications as TR after OHT. Alternative methods, if properly validated, such as IMEG (intramyocardial electrocardiography) and AlloMap test, would be hoped for rejection surveillance allowing reduction for the need and frequency of EBM [1].
The median interval for the development of TR after OHT was 81 months after transplantation and the mean time from the last EMB to cardiac surgery was 67±52.7 months suggesting a late referral for surgery. Indeed all patients had a right ventricular dilatation and dysfunction, high central venous pressure and low cardiac index, so the presence of right ventricular dysfunction maybe underestimated preoperatively might explain the high rate of mortality and complications in our series. Since tricuspid surgery has been reported to be associated with poor prognosis in patients with right ventricular dysfunction [6,8,10], better results would be achieved with early surgery and more appropriate evaluation of right ventricular function by echocardiography or nuclear magnetic resonance. In fact, other series with a more precocious referral reported lower mortality and morbidity following tricuspid surgery after OHT [2,4].
The optimal surgical approach to treat TR in cardiac transplant patients remains unclear [3,11], there are no specific reports describing the outcomes of tricuspid valve repair or replacement in the setting of right ventricular failure when a surgical technique comprising minimally invasive approach and beating heart is adopted.
During beating-heart valve surgery, the heart is kept empty and myocardial protection is achieved through continuous coronary perfusion decreasing the ischemia-reperfusion injury that follows standard manoeuvres of aortic cross-clamping [11-14]. Despite these preventive measures a drug refractory cardiogenic shock occurred in 2 cases. Even the prompt employment of ECLS was ineffective in allowing recovery of cardiac function and adequate organ perfusion. Reoperation on tricuspid valve via thoracotomy without aortic clamping can also be useful in cases of suspected contact between sternum and aorta or right ventricle, porcelain aorta or dense pericardial adhesion. This approach can reduce the risk of infections (medistinitis and osteomielitis) and bleeding. The described surgical technique permitted excellent exposure of the tricuspid valve, very limited bleeding, no infections and low level of troponin as marker of good cardiac preservation.
Concerning the decision of repairing or replacing the valve, Filsoufi et al. suggested valve repair only in presence of tricuspid regurgitation secondary to annular dilatation viceversa in patients with biopsy-related reccomended leaflet prolapse, a bioprosthetic valve replacement because of potential late repair failure [4]. Wahlers et al. reported superior outcome among 8 patients with TVR compared to 4 with valve repair [1]. In our series both TVR and TVRP were employed, with similar results and no failure of bioprostesis or repair was detected at follow up.
In conclusion the surgical treatment of TR associated with right ventricular dysfunction after heart transplantation showed high morbidity and mortality even if minimally invasive approach was employed. More careful assessment of right ventricular function is recommended to define the best timing for surgery.
References
- Wong RC, Abrahams Z, Hanna M, Pangrace J, Gonzalez-Stawinski G, et al. (2008) Tricuspid regurgitation after cardiac transplantation: an old problem revisited. J Heart Lung Transplant 27: 247-252.
- Alharethi R, Bader F, Kfoury AG, Hammond ME, Karwande SV, et al. (2006) Tricuspid valve replacement after cardiac transplantation. J Heart Lung Transplant 25: 48-52.
- Chan MC, Giannetti N, Kato T, Kornbluth M, Oyer P, et al. (2001) Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant 20: 709-717.
- Filsoufi F, Salzberg SP, Anderson CA, Couper GS, Cohn LH, et al. (2006) Optimal surgical management of severe tricuspid regurgitation in cardiac transplant patients. J Heart Lung Transplant 25: 289-293.
- Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, et al. (2010) European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 11: 307-332.
- Aziz TM, Burgess MI, Rahman AN, Campbell CS, Deiraniya AK, et al. (1999) Risk factors for tricuspid valve regurgitation after orthotopic heart transplantation. Ann Thorac Surg 68: 1247-1251.
- Berry GJ, Angelini A, Burke MM, Bruneval P, Fishbein MC, et al. (2011) The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: evolution and current status (2005-2011). J Heart Lung Transplant 30: 601-611.
- Nath J, Foster E, Heidenreich PA (2004) Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardio 43: 405-409.
- Stahl RD, Karwande SV, Olsen SL, Taylor DO, Hawkins JA, et al. (1995) Tricuspid valve dysfunction in the transplanted heart. Ann Thorac Surg 59: 477-480.
- Badano LP, Muraru D, Enriquez-Sarano M (2013) Assessment of functional tricuspid regurgitation. Eur Heart J 34: 1875-1885.
- Pinney SP (2012) The role of tricuspid valve repair and replacement in right heart failure. Curr Opin Cardiol 27: 288-295.
- Salhiyyah K, Taggart D (2009) Beating-heart valve surgery: A systematic review. Asian Cardiovasc Thorac Ann 17: 650-658.
- Wang J, Liu H, Xiang B, Li G, Gruwel M, et al. (2006) Keeping heart empty and beating improves preservation of hypertrophied hearts for valve surgery. J Thorac Cardiovasc Surg 132: 1314-1320.
- Turer AT, Hill JA (2010) Pathogenesis of myocardial ischemia reperfusion injury and rationale for therapy. Am J Cardiol 106: 360-368.