Journal of Chemistry and Applications
Download PDF
In conclusion, a comparative studies of application a new catalyst - Ce(III) supported on weekly acidic cation exchanger resin and catalysts based on Ce(III) chloride, eventually doped by sodium iodide and/or supported on silica gel, were applied to one-pot syntheses of α-aminophosphonates, and 4-amidotetrahydropyrane derivatives. The efficiency of all applied catalytic systems depends on the electron character of the present functional group in the starting 4-X-benzaldehydes, low reactive methoxy-substrate are activated very effectively. Further, it was found that the Ce(III) cation supported on the resin provided at least as effective as other catalytic systems used so far and moreover takes advantage in simple separation and reusing more than ten times without loss of the catalytic efficiency. Solid support of Ce(III) cation was realized with low-cost industrial resin resulting in further savings. The used organic resin has not abrasive effect on surface of reaction vessels, in contradistinction to silica gel carrier. Finally, the advantages of the present protocol make the procedure an attractive alternative to the existing methods for the synthesis of α-aminophosphonates, and 4-amidotetrahydropyrane derivatives.
Research Article
*Address for Correspondence: Pavel Pazdera, Centre for Syntheses at Sustainable Conditions and Management, Department of Chemistry, Masaryk University, Kamenice 5, CZ-625 00 Brno, Czech Republic, E-mail: pazdera@chemi.muni.cz
Citation: Havránková E, Pazdera P. Kabachnik-Fields and Prins-Ritter Synthesis: Application of Ce(III) Supported on a Weakly Acidic Cation-exchanger Resin in Comparative Study. J Chem Applications. 2015;2(1): 6.
Copyright © 2015 Havránková E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.Journal of Chemistry & Applications | ISSN 2380-5021 |Volume: 2, Issue: 1
Submission: 05 February 2015 | Accepted: 16 March 2015 | Published: 21 March 2015
Reviewed & Approved by: Dr. Xinyan Wang, Associate Professor, Department of Chemistry, Tsinghua University, China
Keywords: α-Aminophosphonates; 4-Amidotetrahydropyranes; Metal catalysis; Polymer supported Ce(III); Solid support catalysis
Materials
Kabachnik-Fields and Prins- Ritter Synthesis: Application of Ce(III) Supported on a Weakly Acidic Cation-exchanger Resin in Comparative Study
Eva Havránková and Pavel Pazdera*
- Centre for Syntheses at Sustainable Conditions and Management, Department of Chemistry, Masaryk University, Kamenice 5, Czech Republic
*Address for Correspondence: Pavel Pazdera, Centre for Syntheses at Sustainable Conditions and Management, Department of Chemistry, Masaryk University, Kamenice 5, CZ-625 00 Brno, Czech Republic, E-mail: pazdera@chemi.muni.cz
Citation: Havránková E, Pazdera P. Kabachnik-Fields and Prins-Ritter Synthesis: Application of Ce(III) Supported on a Weakly Acidic Cation-exchanger Resin in Comparative Study. J Chem Applications. 2015;2(1): 6.
Copyright © 2015 Havránková E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.Journal of Chemistry & Applications | ISSN 2380-5021 |Volume: 2, Issue: 1
Submission: 05 February 2015 | Accepted: 16 March 2015 | Published: 21 March 2015
Reviewed & Approved by: Dr. Xinyan Wang, Associate Professor, Department of Chemistry, Tsinghua University, China
Abstract
We described results of comparative studies of the application of Ce(III) cation supported on macroporous weakly acidic polyacrylate resin as catalyst in two model three-component domino syntheses, i.e. Kabachnik-Fields domino synthesis of α-aminophosphonates, and Prins-Ritter domino synthesis of 4-amidotetrahydropyrane derivatives, respectively. It was discovered that cation Ce(III) supported on a weakacid macroporous cation-exchanger shown in the studied reactions at least the same or better catalytic activity as salt CeCl3.7H2O, eventually doped by NaI. Further, we found that reactivity of the low reactive 4-methoxybenzaldehyde as carbonyl compounds in the presence of Ce(III) catalyst increases, while the reactivity of the highly reactive 4-nitrobenzaldehyde in this case strongly decreases due to its low coordination with the catalyst. Finally, the presented synthetic protocols using cerium(III) supported on low-cost industrial resin further increases green impact of studied domino syntheses of α-aminophosphonates, and 4-amidotetrahydropyrane derivatives.Keywords: α-Aminophosphonates; 4-Amidotetrahydropyranes; Metal catalysis; Polymer supported Ce(III); Solid support catalysis
Introduction
Molecule containing phosphonate structural motives are highly biologically potent. They can be used as inhibitors of synthase [1], HIV protease [2], PTPases [3,4], rennin [5], enzymes [6], antibiotics[7], herbicides [8], etc.Generally α-aminophosphonates can be prepared by addition of phosphorous nucleophiles to imines in the presence of acids [9-11] or in three-component domino synthesis of amines, aldehydes and triethylphosphites with Lewis and Brønsted acids as catalysts. In this case, as catalysts can be used FeCl3 [12], ZrCl4 [13], SbCl3/Al2O3 [14], Amberlyst-15 [15], sulfamic acid [16], BF3.Et2O [17], etc.
4-Amidotetrahydropyrane derivatives are often present in natural products as a glycamino acid[18,19], ambruticins VS [20,21] and others. 4-Amidotetrahydropyrane derivatives can be generally prepared by the Prins-Ritter synthesis [22-24].
At the turn of the century lanthanide Lewis acids, as cerium(III) chloride (CeCl3.7H2O), have attracted an attention in organic synthesis due to their high reactivity, stability, ease of handling, air tolerance, low toxicity and low cost [25,26]. Cerium(III) is the most commonly used as chloride or nitrate salt itself or supported on silica gel eventually doped by sodium iodide-this type of supported catalyst was developed by Bartoli and Marcantoni [27,28].
Recently, we published two articles which dealt with the application Ce(III) cation supported on macroporous weakly acidic polyacrylate resin as catalyst for domino syntheses of some nitrogen containing heterocycles [29] and for synthesis of imines [30] in comparison with CeCl3.7H2O, eventually doped by sodium iodide.The obtained results showed that the polymer supported Ce(III) provides at least comparable or better catalytic activity in comparison with inorganic Ce(III) salts mentioned above.
Solid supported catalysts combine the advantages of both heterogeneous and homogenous catalysts [31,32]. They have high selectivity, reactivity, and stability. They are easily separable and recyclable. The one-pot and namely domino syntheses, by minimizing the number of intermediate synthetic steps and therefore minimizing the amount of waste, are very suitable type of syntheses for Green Chemistry [33].Due to these characteristics they are suitable for reactions carried ut in accordance with the goals and principles of Green Chemistry [33].
Experimental Section
All reagents were purchased from commercial suppliers and used as received without further purification. Purolite C 104 Plus (Purolite® Worldwide), i.e. weakly acidic polyacrylic cation-exchanger resin of macroporous type, H+ ionic form, total volume capacity 4.5 eq/L,specific gravity 1.19 g/mL, was used as solid support.
All the reactions were monitored by TLC performed on precoated Silica gel 60 F254 plates (Merck). Synthesis of α-aminophosphonates: ethanol was used as eluent; UV light (254 and 356 nm) and ninhydrine reagent were used for detection of spots at 180 °C. Synthesis of 4-amidotetrahydropyranes: Et2O was used as eluent; UV light (254 and 356 nm) was used for detection of spots.
All the reactions were monitored by TLC performed on precoated Silica gel 60 F254 plates (Merck). Synthesis of α-aminophosphonates: ethanol was used as eluent; UV light (254 and 356 nm) and ninhydrine reagent were used for detection of spots at 180 °C. Synthesis of 4-amidotetrahydropyranes: Et2O was used as eluent; UV light (254 and 356 nm) was used for detection of spots.
All products were identified by use NMR and IR spectral methods, and by comparing of the measured melting points with literature values. 1H-NMR and 13C-NMR spectra were recorded on DRX 300 Avance (Bruker Biospin) spectrometer using tetramethylsilane as an internal standard. Melting points are uncorrected and were recordedon Kofler’s block Boetius Rapido PHMK 79/2106 (Wägetechnik), temperature gradient 4 °C min-1.
Catalyst preparation
Ce(III) cations supported on cation exchanger:The catalytic system containing Ce(III) cations supported on a weakly acidic macroporous cation exchanger of polyacrylate type was prepared according to the patent [34]. Purolite C 104 Plus (75 g) was suspended in 200 mL of water and a saturated aqueous potassium carbonate solution was added under stirring until pH of the solution remained at value of 12 for 10 min after the last addition. Then was the aqueous solution was decanted and the resin beads were washed 4 times by 200 mL of water. Cerium(III) chloride heptahydrate (122.7 g, 33 mmol) was dissolved in 500 mL of water and modified resin beads were dropped into the solution which was then stirred overnight. Then was the aqueous solution was again decanted and the resin beads were washed 2 times by 200 mL of water and 2 times by methanol and finally dried in vacuum to constant weight. The prepared catalyst has cerium content about 2.3 mmol of Ce(III) per 1 g of modified resin beads [34]. This catalyst is also available from TauChem Ltd., Bratislava, Slovakia, http://www.tau-chem.sk/en/About/Companydescription. alej.
General synthetic proceduresGeneral procedure for preparation of α-aminophosphonates [35]: A mixture of 2 mmol of amine, 2.2 mmol of diethyl phosphite, and 2 mmol of aldehyde with 0.06 mmol of appropriate catalytic system (Table 1) in 5 mL of ethanol as a solvent, was stirred at room temperature until disappearance of amine from the reaction mixture. The reaction was monitored by TLC. After the completion of reaction, the reaction mixture was heated until the precipitated crystals dissolved. Than the product was precipitated by adding ice water, filtrated off and washed with cold water. Obtained 1H, 13C NMR and FT IR spectral data of all synthesized products were in agreement with published one [35].
General procedure for preparation of 4-amidotetrahydropyranes[36]: A mixture of 1.2 mmol of but- 3-en-1-ol, 1 mmol of 4-X-benzaldehyde, and 1.5 mmol of acetyl chloride, in role of activator for Prins-Ritter domino synthesis [36], with 0.05 mmol of appropriate catalytic system (Table 2) in 5 mL of acetonitrile as a solvent and nucleophilic reagent, was stirred atroom temperature until disappearance of carbonyl compound from the reaction mixture. The reaction was monitored by TLC. After the completion of reaction, the reaction mixture was quenched with water and extracted with 2x10 mL of ethyl acetate. The combined organic layers were dried with anhydrous Na2SO4 and purified with silica gel Acquired 1H, 13C NMR and FT IR spectral data of obtained products were in accordance with published one [36].
To prove the efficiency of the catalytic system of Ce(III) cations supported on weakly acidic macroporous cation exchanger polyacrylate type, we performed a comparative study on two model domino syntheses.
Results and Discussion
We verified the both model synthetic procedures with published catalysts according to the general procedure published in the articles [35,36]. Syntheses were then carried out with Ce(III) supported on above described resin and the results were compared in terms of the reaction time and the yield relative to the used catalytic system.
Results of α-aminophosphonates study revealed that the Ce(III) cation supported on the resin provided at least as satisfying results as other catalytic systems, the results in Table 1 show the greatest shortening of reaction times and the maximal yields for the catalytic system Ce(III) supported on the weakly acidic cation exchanger in the comparison with the another used Ce(III) catalysts (Table 1). On the other hand, we observed that the electron effect of the present substituent on the used 4-X-benzaldehyde (X=OCH3, H, NO2) is under catalysis changed. In accord to results obtained and explained in article [29,30]. We found also that positive role of used catalyst is more significant for electron donating group in typically low reactive 4-methoxybenzaldehyde in contrast with very good reactive 4-nitrobenzaldehyde. The electron influence of substituents in the applied 4-Y-anilines (Y=OH, H, Cl) is not significant because of weak coordination/affinity between nitrogen atom of aniline amino group and Ce(III) – lower reactivity of 4-hydroxyaniline may be probably evoked by coordination of phenolic oxygen with Ce(III).
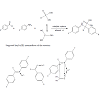
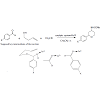
The role of Ce(III) cations as a catalyst in this procedure can be explained by formation of the some supposed key intermediates (Figure 1).
In the case of syntheses of 4-amidotetrahydropyrane derivatives, the reactions catalyzed by Ce(III) ions in the presence of acetyl chloride as co-activator were in general much faster and yields were higher than in the case of uncatalyzed reactions, similarly as in the previous model syntheses. The highest yields and the shortest reaction times were again observed when procedures were catalyzed by catalytic system Ce(III) supported on the weakly acidic cation exchanger. Details are demonstrated in (Table 2).
Acceleration of the reaction in the presence of Ce(III) cations can be explained by the supposed key intermediates formation in the course of reaction, as well (Figure 2). Coordination of Ce(III) cation to the carbonyl oxygen of the used 4-X-benzaldehydes (X=OCH3, H, NO2) and raises electron deficiency on this group and thus facilitates the bond formation between the oxygen of but-3-en-1-ol and the carbon of benzaldehyde group under hemiacetal formation (See Figure 2, for mechanism detail the ref. [36]). The electron effect of substituent X in the used 4-X-benzaldehydes on the reaction results was the same as described above for α-aminophosphonate formation.
Conclusions
References
- Sikorski JA, Miller MJ, Braccolino DS, Clearyb DG, Coreyb SD, et al. (1993) EPSP synthase - the design and synthesis of bisubstrate inhibitors incorporating novel 3-phosphate mimics. Phosphorus Sulfur Silicon Relat Elem 76: 375-378.
- Stowasser B, Budt KH, Jian-Qi L, Peyman A, Ruppert D (1992) New hybrid transition state analog inhibitors of HIV protease with peripheric C2-symmetry. Tetrahedron Lett 33: 6625-6628.
- Bruke TR, Barchi JJ Jr, George C, Wolf G, Shoelson SE, et al. (1995) Conformationally constrained phosphotyrosyl mimetics designed as monomeric src homology 2 domain inhibitors. J Med Chem 38: 1386-1396.
- Bruke TR, Kole HK, Roller PP (1994) Potent inhibition of insulin receptor dephosphorylationby a hexamer peptide containing the phosphotyrosyl mimetic F2pmp. Biochem Biophys Res Commun 204: 129-134.
- Patel DV, Rielly-Gauvin K, Ryono DE (1990) Preparation of peptidic α-hydroxy phosphonates a new class of transition state analog renin inhibitors. Tetrahedron Lett 31: 5587-5590.
- Peyman A, Budt KH, Paning JS, Stowasser B, Ruppert D (1992) C2-symmetricphosphinic acid inhibitors of HIV protease. Tetrahedron Lett 33: 4549-4552.
- Atherton FR, Hassall CH, Lambert RW (1986) Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonicacid and (aminomethyl)phosphonic acid. J Med Chem 29: 29-40.
- Kafarski P, Lejczak B, Mastalerz P, Duś D, Radzikowski C (1985) N-(Phosphonoacetyl)amino phosphonates. phosphonate analogues of N-(phosphonoacetyl)-L-aspartic acid (PALA). J Med Chem 28: 1555-1558.
- Laschat S, Kunz H (1992) Carbohydrates as Chiral templates: stereoselective synthesis of (R)- and (S)-α-aminophosphonic acid derivatives. Synthesis 1992: 90-95.
- Kudzin ZH, Lyzwa P, Luczak J, Andrijewski G (1997) 1-Aminoalkanephosphonates. Part II. A facile conversion of 1-aminoalkanephosphonic acids into 0,0-diethyl 1-aminoalkanephosphonates. Synthesis 1997: 44-46.
- Lee SG, Park JH, Kang J, Lee JK (2001) Lanthanide triflate-catalyzed three component synthesis of α-aminophosphonates in ionic liquids. a catalyst reactivity and reusability study. Chem Commun 1698-1699.
- Ranu BC, Hajra A, Jana U (1999) General procedure for the synthesis of α-aminophosphonates from aldehydes and ketones using indium (iii) chloride as a catalyst. Org Lett 1: 1141-1143.
- Rezaei Z, Firouzabadi H, Iranpoor N, Ghaderi A, Jafari MR, et al. (2009) Design and one-pot synthesis of α-aminophosphonates and bis(α-aminophosphonates) by iron(iii) chloride and cytotoxic activity. Eur J Med Chem 44: 4266-4275.
- Ambica S, Kumar SC, Taneja MS, Hundal K, Kapoor K (2008) One-pot synthesis of α±-aminophosphonates catalyzed by antimony trichloride adsorbed on alumina. Tetrahedron Lett 49: 2208-2212
- Tajbakhsh M, Heydari A, Alinezhad H, Ghanei M, Khaksar S (2008) Coupling of aldehydes, amines, and trimethylphosphite promoted by amberlyst-15: Highly efficient synthesis of α-aminophosphonates. Synthesis 2008: 352-354.
- Mitragotri SD, Pore DM, Desai UV, Wadgaonkar PP (2008) Sulfamic acid: An efficient and cost-effective solid acid catalyst for the synthesis of α-aminophosphonates at ambient temperature. Catal Commun 9: 1822-1826.
- Ha HJ, Nam GS (1992) An efficient synthesis of anilinobenzylphosphonates. Synth Commun 22: 1143-1148.
- Suhara Y, Yamaguchi Y, Collins B, Schnaar RL, Yanagishita M, et al. (2002) Oligomers of glycamino acid. Bioorg Med Chem 10: 1999-2013.
- Höfle G, Steinmetz H, Gerth K, Reichenbach H (1991) Antibiotics from gliding bacteria, XLIV. Ambruticins VS: New members of the antifungal ambruticin family from Sorangium cellulosum. Liebigs Ann Chem 1991: 941-945.
- Snider BB, Hawryluk N (2000) Synthesis of (-)-Dysiherbaine. Org Lett 2: 635-638.
- 21. Michelet V, Genet JP (2005) An overview of synthetic and biological studies of ambruticin and analogues. Curr Org Chem 9: 405-418.
- Yadav JS, Reddy MS, Rao PP, Prasad AR (2006) Stereoselective synthesis of anti-1,3-Diol units via prins cyclisation: application to the synthesis of (α)-Sedamine. Tetrahedron Lett 47: 4397-4401.
- Yadav JS, Reddy MS, Prasad AR (2006) Stereoselective synthesis of polyketide precursors containing an anti-1,3-Diol system via a prins cyclisation and reductive cleavage sequence. Tetrahedron Lett 47: 4937-4941.
- Yadav JS, Reddy MS, Prasad AR (2006) Stereoselective synthesis of (α)-tetrahydrolipstatin via Prinscyclisations. Tetrahedron Lett 47: 4995-4998.
- Molander GA (1992) Application of lanthanide reagents in organic synthesis. Chemical Reviews 92: 29-68.
- Imamoto T (1994) Lanthanides in organic synthesis, XVII Ed. Academic Press, San Diego, USA.
- Bartoli G, Bosco M, Marcantoni E, Petrini M, Sambri L, et al. (2001) Conjugate addition of amines to α,β-Enones promoted by CeCl3.7H2O−NaI system supported in silica gel. J Org Chem 66: 9052-9055.
- Bartoli G, Bartolacci M, Bosco M, Foglia G, Giuliani A, et al. (2003) The michael addition of indoles to α,β-unsaturated ketones catalyzed by CeCl3.7H2O−NaI combination supported on silica gel. J Org Chem 68: 4595-4597.
- Havránková E, Pazdera P (2014) Comparative studies of catalytic application of cerium(iii) chloride and resin supported cerium(iii) in domino syntheses of 1,5-benzodiazepines and 1,3-diazine skeletons. J Chem Eng Chem Res 1: 229-237.
- Havránková E, Pospisil P, Pazdera P (2014) Synergism of metal and organocatalysis in condensation reactions of aromatic aldehydes with anilines affording imines: effect of catalysts on the base of a supported cerium(iii) and proline. Sci J Chem 2: 1-8.
- Choplin A, Quignard F (1998) From supported homogeneous catalysts to heterogeneous molecular catalysts. Coord Chem Rev 178-180: 1679-1702.
- Ahluwalia VK, Aggarwal R (2006) Organic synthesis: special techniques, 2nd ed, alpha science international: Oxford, England.
- Pazdera P (2011) Chapter 1. Emerging ubiquity of green chemistry in engineering and technology, in handbook on applications of ultrasound-sonochemistry for sustainability, 1st ed. CRC Press/Taylor & Francis Group: Boca Raton FL, USA.
- Pazdera P, Zberovská B, Němečková D, Datinská V (2013) Catalyst based on d-metal complex for chemical syntheses and process for preparing thereof. CZ 20110799 (A3).
- Jafari AA, Nazarpour M, Abdollahi-Alibeik M (2010) CeCl3•7H2O-catalyzed one-pot Kabachnik–Fields reaction: A green protocol for three-component synthesis of α-aminophosphonates. Heteroatom Chemistry 21: 397-403.
- Yadav JS, Reddy BV, Narayanakumar GG KS, Madhusudhan Reddy G (2007) CeCl3•7H2O/AcCl-catalyzed prins–ritter reaction sequence: a novel synthesis of 4-amido tetrahydropyrane derivatives. Tetrahedron Lett 48: 4903-4906.