Journal of Andrology & Gynaecology
Download PDF
Review Article
The Developmental Process of Spermatogenesis
Dalia K1, Ali K2 and Ghina G1*
1Department of Obstetrics and Gynecology, American University of
Beirut Medical Center, Lebanon
2Division of Gynecologic Oncology, American University of Beirut
Medical Center, Lebanon
*Address for Correspondence: Ghazeeri G, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics & Gynecology, American University of Beirut
Medical Center, Hamra, Beirut, PO BOX: 113-6044 Lebanon, Telephone: 01-350000, E-mail: gg02@aub.edu.lb
Submission: 25 September, 2019
Accepted: 9 October, 2019
Published: 18 October, 2019
Copyright: © 2019 Dalia K. This is an open access article distributed
under the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided
the original work is properly cited.
Abstract
The multiplication and development of germ cells in the
seminiferous tubules of the testiclesoccur through a complex series of
cellular events that are controlled by multiple signals. It is composed of
6 stages in humans (Figure 1).
Spermatogonial stem cells are self-renewed via mitosis, meiosis
and contribute to the formation of haploid spermatids from diploid
spermatocytes. Through the process of spermiogenesis, spermatids
undergo maturation and are transformed into functional spermatozoa
which are released at spermiation after the breakage of intercellular
bridges attaching the spermatids to Sertoli cells. Spermatogenesis is
a continuous process requiring the contribution of numerous cell and
regulatory factors. Its understanding is essential in order to advance
research for treatment of male infertility. The different stages of
spermatogenesis along with the main roles of Sertoli cell and BTB will
be reviewed. Some emerging fields in research regarding the new
classification was briefly examined for a better understanding of the
complexity of the process.
Keywords
Spermatogenesis; Spermiation; Spermiogenesis; Sertoli
cells
Introduction
Spermatogenesis is a process occurring in the Seminiferous
Tubules (ST) of the testicles. The multiplication and development of
germ cells occur through a complex series of cellular events that are
controlled by multiple signals. While fourteen stages are found in rats,
the seminiferous epithelial cycle is composed of 6 stages in humans
[1]. Spermatogonial stem cells are self-renewed via mitosis, meiosis
(I and II) and contribute to the formation of haploid spermatids
from diploid spermatocytes. Through the process of spermiogenesis,
spermatids undergo maturation and are transformed into functional
spermatozoa which are released at spermiation after the breakage of
intercellular bridges attaching the spermatids to Sertoli cells [2,3].
Intra-testicular and extra-testicular regulatory hormones involving
the release of Follicle Stimulating Hormone (FSH) from pituitary and
testosterone from leydig cells are the prime components of a wellorganized spermatogenesis [4]. Sertoli cells control the milieu within
the seminiferous tubules in order to facilitate the progression of germ
cells to spermatozoa. Hense, spermatogenesis is regulated through
the control of FSH on Sertoli cells and LH on Leydig cells.
The seminiferous epithelium and hormonal regulation:
The testis is composed of 400 to 600 ST, which is the functional
unit of the test is where 300 million sperms per day are produced
after puberty [5]. Sertoli cells have a significant role in supporting the
growth of the gonadal cells and thus are known as the ‘nurse’ cells
[6]. Leydig cells in return, under the influence of LH, supports the
production of testosterone necessarily for the step by step process of spermatogenesis [7].The Blood-Testis Barrier (BTB) is situated within the basement
membrane of the ST, providing the microenvironment for the
emergence of spermatids [8]. This barrier is well established at
puberty, concomitantly with the onset of meiosis [9]. The basal and
the ad-luminal compartments are the compartments found in the
Seminiferous Epithelium (SE) which are separated by the BTB. The
basal compartment includes undifferentiated spermatogonia (A and
B) and preleptotene spermatocytes. The ad-luminal compartment
is inhabited by the primary, secondary spermatocytes, haploid
spermatids and spermatozoa. During the 6 stages of spermatogenesis,
the junctions between Sertoli cells and reproductive cells are in a
constant remodeling process to allow transportation through the
SE. It is in the ad-luminal compartment that meiosis I and II, the
formation up to the spermatozoa stage and spermiation take place
[10].
The different stages of the epithelial cycle:
In humans, spermatogonia enter spermatogenesis every 16
days, divide in a continuous way through mitosis to produce
different variety of cells. This is entitled “cycle of the seminiferous
epithelium” [11]. The detail inspection of cross sections of tubules
over the years uncovered 6 main stages of spermatogenesis or cellular
associations [12].Cycle of the Seminiferous Epithelium
The epithelial cycle is composed of 6 different stages along the
SE of the testis [13]. A stage refers to a specific segment of the SE
over time [14]. The duration of spermatogenesis is around 64 days in
which a type A spermatogonia is transformed into multiple haploid
spermatozoa [15]. In this context, an epithelial cycle takes 16 days to
be completed, yet it takes four cycles for a spermatogonia to become
a spermatid along the section of a tubule (74 days).
Spermatogonial proliferation and spermatocytes formation:
Two types of spermatogonia (A and B) are present in the basal compartment of the ST. Spermatogonia Ad (A dark type) and Ap
(A pale type) represent type A spermatogonia. Spermatogonia
Ap have a self-renewal property and thus are predominant while
spermatogonia Ad are observed as the regenerative reserve of stem
cells [12,16]. On the other hand, B type spermatogonia characterize
the beginning of reproductive cell growth up to the spermatids stage.
The multiplication of spermatogonia is synchronous through mitosis
where the divided cells communicate via cytoplasmic bridges that
dissolve at the spermatids stages [17].
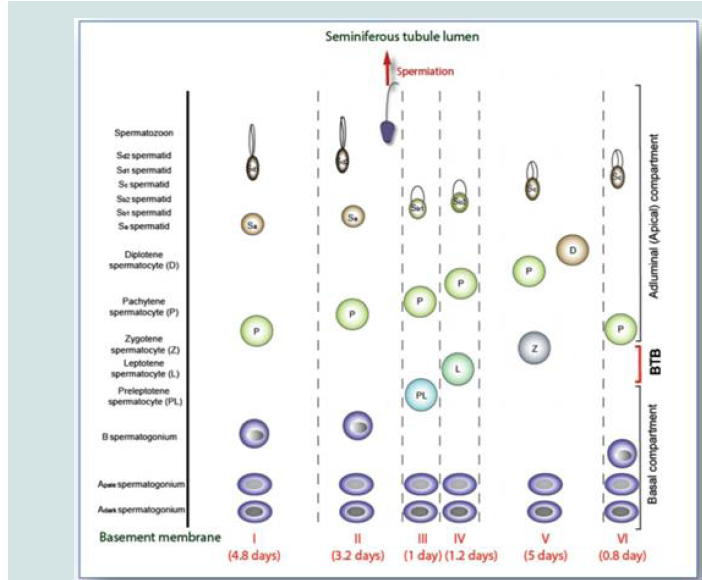
Figure 1: The six stages of the seminiferous epithelial cycle (I-VI) in the human testis (Picture copied from Chen et al. 2017).
The multiplication and differentiation of these cells is regulated by
the tyrosine kinase receptor (cKIT) protein which is only manifested
in spermatogonia, round spermatids and spermatozoa. Its ligand
(cKIT ligand) is found at the level of Sertoli cells [18]. Retinoic acid
is involved in the commencement of meiosis and the conversion of
undifferentiated spermatogonia into type A spermatogonia [19].
Data in the literature is inconsistent about the stage of appearance
of spermatogonia B. Clermont documented that that these cells were
formed between stages VI and I, evident in stages I and II and divide
into preleptotene spermatocytes in the late stage II [11]. After the
ultimate spermatogonial division, cells undergo 2 stages of meiosis.
A pair of spermatogonia Ap produces 8 preleptotene spermatocytes
[20].
Stage III of the epithelial cycle represents the differentiation
of spermatogonia B into preleptotene spermatocytes, which are
shifted past the BTB while converting to leptotene spermatocytes.
Thus, after the initiation of meiosis at puberty, spermatocytes are
located in the SE at different stages: leptotene, zygotene, pachytene,
and diplotene [21]. During meiosis I, diplotene spermatocytes are
transformed into secondary spermatocytes (1N:2C) which are then
transformed into two round spermatids (1N:1C) via meiosis II. To
note that primary spermatocytes start their meiosis in the basal
compartment of SE at the leptotene stage and reach the adluminal
compartment through the BTB to proceed with further stages of
division into secondary spermatocytes. Meiosis I involve DNA
replication, condensation of chromosomes, pairing and crossing over of homologous chromosomes [17]. Lactate is the main energy
source of spermatocytes which is metabolized by the Sertoli cells
via glucose transporters (GLUT1). This mechanism is controlled by
a close regulation between gonadotrophins, steroids and paracrine
factors [22].
Spermatids, spermiogenesis and spermiation:
Four haploid spermatids are the products of the two meiotic
divisions of each spermatocyte.Spermiogenesis is the process during which spermatids are
transformed into spermatozoa. Different techniques of staining were
used over the years to describe the formation of human spermatids
[11]. Nuclear characteristics of spermatids were described by Holstein
& Roosen by using glutaraldehyde fixation and toluidine blue stain.
Subsequently, they were able to detect 6 main stages (Sa, Sb1, Sb2, Sc,Sd1, and Sd2) including morphological changes during which an
acrosomic granule is produced in the Golgi apparatus and grows
over the nucleus. Additional changes happen, including: acrosome
formation, condensation of nuclear chromatin, detachment of
the cytoplasm forming the residual body, which is subsequently
digested by Sertoli cells via phagocytosis, and finally the formation
of a mature spermatozoon [23]. Any disruption at the level of the
acrosome formation, nucleus and/or flagellum maturation may lead
to maturational arrest or hypospermatogenesis [24]. All these stages
are still within the premises of the human testis.
Spermiation is the final process during which mature spermatozoa
are delivered into the lumen of ST for their subsequent maturation in
the epididymis. The delivery of matured spermatids is achieved via
the Sertoli cells during which they separate from their connections
and become spermatozoa [25]. Residual bodies including parts
of the spermatids are digested into the Sertoli cells. The molecular
mechanism is still poorly understood yet, it is dependent on FSH and
testosterone [26].
Spermatozoa:
The final product of spermatogenesis is the spermatozoa. The
unique shape of spermatozoa permits its precise contact with the
oocyte: nucleus is condensed, protected by an acrosome and attached
to a flagellum to allow progressive motility. The motility is acquired
during the transport in the epididymal ducts, depending on the
normal development of axonemes, presence of mitochondria and
implantation of flagellum at the nucleus [23]. To note that the highly
condensed spermatozoa has a particular form of DNA packaging in
the sperm nuclei where loops are packed as doughnuts, thus allowing
the transfer of the genome in a compacted form to the zygote [27].Generations of differentiated germ cells are illustrated from the
basement membrane upward to the tubule lumen. Spermatogonia
are established in stage V, present in all cellular associations. The
preleptotene spermatocytes appear during stage III, are transported
through the BTB, to undergo meiosis during the stage IV of the
cycle. Newly formed spermatids are present in stage I with spherical
nucleus. It is till the end of stage II that spermiation is noted.
Different methods were used to precisely characterize the
morphology of germ cells within the SE. The present descriptions
are based on initial studies done during the early 60s [11]. Although, these were innovative studies, they had flaws in describing the nuclear
features of germ cells and the organization among the different stages.
For a better understanding of the dynamics of spermatogenesis, it has
been recently revisited by several investigators using high resolution
light microscopy method on open testes biopsies from adult and
elderly patients. They proposed new cellular associations in order
to enable a more advanced and consistent source of reference [14].
The number of six stages originally proposed was maintained, yet the
stages were better defined.
Conclusion
Spermatogenesis is a continuous process requiring the
contribution of numerous cell and regulatory factors. Its
understanding is essential in order to advance research for treatment
of male infertility. Numerous areas of the ST are occupied by different
stages of spermatogenesis; hence several stages can be discerned in
an isolated tubule section. The different stages of spermatogenesis
along with the main roles of Sertoli cell and BTB were reviewed.
Some emerging fields in research regarding the new classification was
briefly examined for a better understanding of the complexity of the
process.