Journal of Andrology & Gynaecology
Download PDF
Research Article
Urinary Spot Albumin/Creatinine Ratio for Documenting Proteinuria in Women with Hypertensive Disorders with Pregnancy
Amr Ahmed Abdelrhman* and Ahmed Mahmoud Abdou
- Lecturer of Obstetrics and Gynecology, Faculty of medicine, Zagazig University, Egypt
*Address for Correspondence: Amr Ahmed Abdelrhman, Lecturer of Obstetrics and Gynecology, Faculty of medicine, Zagazig University, Egypt, Tel: +2 01111222964; E-mail: amrabdelrahman@medicine.zu.edu.eg
Citation: Abdelrhman AA, Abdou AM. Urinary Spot Albumin/Creatinine Ratio for Documenting Proteinuria in Women with Hypertensive Disorders with Pregnancy. JAndrol Gynaecol. 2018;6(1): 4.
Copyright: © 2018 Abdelrhman AA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Andrology & Gynaecology | ISSN: 2332-3442 | Volume: 6, Issue: 1
Submission: 11 June, 2018 | Accepted: 13 July, 2018 | Published: 20 July, 2018
Keywords
Proteinuria; Albumin/Creatinine ratio; 24 hour urine protein; Preeclampsia
Abstract
Background: Although, twenty-four hour urine collection was considered the gold standard for quantification of proteinuria, it has many limitations. It is cumbersome for the patient and often inaccurate because of under collection and result availability is delayed for at least 24 hours. The spot albumin creatinine (A/C) ratio use in pregnancy has been extensively studied. Its use has been adopted by some authors as they found that it had been significantly correlated to 24 hour urinary protein estimation while others did not support its use as in cases of preeclampsia kidney function may deteriorate rapidly.
Objective: To evaluate the accuracy of random urine albumin/creatinine ratio as a diagnostic method for quantitative evaluation of proteinuria in hypertensive disorders in pregnant women.
Methods: A total of 70 patients fulfilling inclusion criteria were evaluated with urine albumin/creatinine ratio performed on random voided sample. The entire 24 hour urine sample was collected and analyzed for daily protein excretion. A/C ratio was compared to the 24 hour results.
Results: There was significant positive correlation detected between A/C ratio and 24 hour protein at a cutoff value of 347.35 mg/gm. Area under curve for A/C ratio was 0.730 (P<0.001) showing sensitivity 80.6% and specificity 51.2% to detect protein excretion of 300 mg/24 hr. While, there was significant negative correlation detected between A/C ratio and urine creatinine.
Conclusion: Random A/C ratio could be used as a rapid, easy and reliable test for diagnosis of significant proteinuria in hypertensive disorders with pregnancy, so it can substitute 24 hour urinary protein collection.
Introduction
Preeclampsia is a multisystem disease defined as gestational hypertension with proteinuria or defective placental angiogenesis so, the quantification of proteinuria in preeclampsia is mandatory for diagnosis of the disease [1].
Significant proteinuria is defined by 24 hour urinary protein more than 300 mg/dl or persistent 30 mg/dl in random urine samples [2]. Twenty-four hour urine collection, although the gold standard for estimation of proteinuria has some constraints. It is cumbersome for the patient and often inaccurate because of under collection, and result is delayed for at least 24 hours also [3].
The degree of proteinuria show significant fluctuation throughout the day, even in severe cases. Therefore, significant proteinuria cannot be detected by a single random urinary sample [3].
The spot albumin creatinine (A/C) ratio use in pregnancy has been extensively studied. Its use has been adopted by some authors as they found that it had been significantly correlated to 24 hour urinary protein estimation and in cases of preeclampsia kidney function may deteriorate rapidly while others did not support its use [4].
Both spot albumin/creatinine ratio and spot protein/creatinine ratio had been thoroughly evaluated and used in non-pregnant women. The National Kidney Foundation adopted these tests to evaluate proteinuria in most conditions instead of 24 hour urine collection [5].
The Society of Obstetricians and Gynecologists of Canada, the Society of Obstetric Medicine of Australia and New Zealand and the International Society for the Study of Hypertension in Pregnancy have adopted the spot urine ACR as a practical method for the detection of significant proteinuria (≥0.3 gm/24 hr.) during pregnancy, but this is not proved by other international groups like the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy and American Congress of Obstetricians and Gynecologists [6].
The aim of this study is to evaluate the accuracy of spot urine albumin/creatinine ratio as a diagnostic method for quantitative assessment of proteinuria in hypertensive disorders in pregnant women.
Patients and Methods
Seventy pregnant women with hypertensive disorders with pregnancy were included in the study at the department of Obstetrics and Gynecology, Zagazig University Hospitals from January 2016 to June 2017. An informed signed consent about the aim of the study, the required procedure and the follow-up plan was obtained from each woman. We exclude patients with a known kidney disease, Presence of bacteriuria, Pregestational and gestational diabetes mellitus, women delivered their babies during the urine collection day. All pregnant women who were included in the study were subjected to:
1. Full history taking, which included personal history, obstetric history, menstrual history, present history, previous and present medical diseases (cardiac, hepatic, renal, endocrinal, pulmonary) and family history of diabetes mellitus and hypertension.
2. Physical examination: Which included vital signs as blood pressure, heart rate, temperature and respiratory rate, abdominal examination of fundal level, Leopard’s maneuver and fetal heart sound auscultation, systemic examination of heart, lungs, lower limbs, plus ophthalmic and neurological examination
3. Investigations: Full blood count, blood and Rh grouping, kidney function tests, urine analysis and culture, measurement of urine protein/creatinine ratio and ultrasound.
Sample collection
All women were asked to void urine into clearly labeled container over 24 hours. We excluded patient who were not able to complete 24 hour urinary collection. The 24 hour collection was started in the morning by spontaneous voiding. Then, collected samples were sent for analysis.
A single voided urine specimen (5 ml) (not first sample) was obtained randomly for the calculation of A/C ratio; they were stored at 4 °C immediately after collection and examined within 24 hours.
Urine albumin was measured in the central laboratories of Zagazig university by the Bradford method (Bio-Rad Protein Assay Kit, BioRad Laboratories) using BSA (Bio-Rad) as a calibrator. The assay was performed manually as described by the manufacturer. Briefly, in a 96-well plate the protein calibrator (BSA in 0, 1, 2, 3, and 4 μg/10 µl of isotonic saline solution) or 10 μl of sample (urine sample or a diluted sample as necessary to assess for parallelism with the calibratory curve) were mixed with 200 μl of protein assay solution diluted with 4 volumes of ultrapure water; after 5 minutes, we measured the absorbance of the assay mixture at 595 nm using an Emax precision microplate reader (molecular devices). The intra- and inter-assay imprecisions (CVs) were ≥2.3%.
Urine creatinine was measured by the modified kinetic Jaffe reaction in a 96-well plate with a filter at 490 nm using Spinreact kits. The intra- and inter-assay CVs were ≥1.2%. The urine albumin: creatinine ratio was obtained by dividing the urinary albumin concentration by the urine creatinine concentration, both expressed in (mg/dl).
Principle of assessment of albumin in urine is to measure the shift in absorption spectrum from 460 to 600 nm of the complex that occurs at acid pH between Pyrogallol Red-Molibdate (PRM) and the basic amino groups of urine. The intensity of the colored complex formed if proportional to the concentration of albumin in the sample.
Statistical analysis
All data were collected, tabulated and statistically analyzed using SPSS version 20.
Continuous Quantitative variables e.g. age, laboratory characteristics were expressed as the mean±SD & median (range), and categorical qualitative variables were expressed as absolute frequencies (number) & relative frequencies (percentage). Validity of the screening test (ACR) was assessed in the terms of sensitivity, specificity, predictive value positive, predictive value negative, Likelihood ratio positive, Likelihood ratio negative and accuracy.
Results
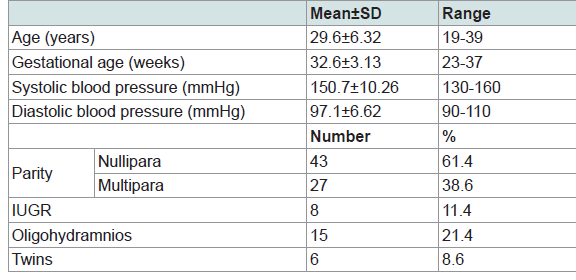
Seventy pregnant women with hypertensive disorders with pregnancy were admitted to Zagazig University hospital during the period from January 2016 to June 2017. Clinical characteristics of study participants are shown in Table 1. The age of females ranged from 19 to 39 years. The gestational ages were found to be ranged between 23 and 37 weeks. It was noticed that nulliparous women were of the highest frequency represented by 61.4% among the studied group.
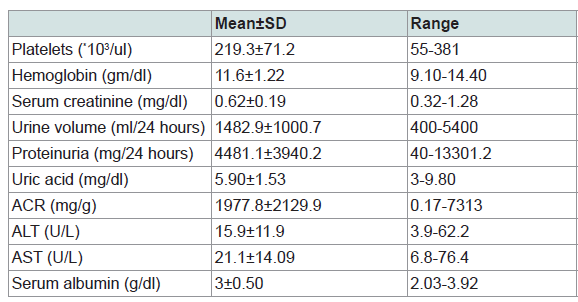
The mean platelet count of the study participants was 219.3*103/ul, the mean hemoglobin level was 11.6 mg/dl, the mean serum creatinine was 0.62 mg/dl, the mean urine volume was 1482.9 ml/24 hs, the mean uric acid was 5.90 mg/dl, the mean protein level in urine was found to be 4481.1 mg/24 hr with range of 40-13301.2 and the albumin/creatinine ratio (ACR) ranged between 0.17 and 7313 with a mean of 1977.8 mg/g. The mean ALT was 15.9 U/L with range of 3.9-62.2, the mean AST was 21.1U/L with range of 6.8-76.4 and serum albumin mean was 3 gm/dl with range of 2.0-3.92 (Table 2).
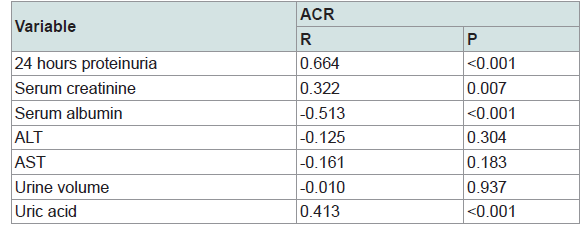
There is a significant positive strong correlation between ACR and 24 hours proteinuria (Figure 1). There is also a significant positive weak correlation between ACR and serum creatinine and uric acid. A weak negative correlation was detected between ACR and serum albumin (Table 3).
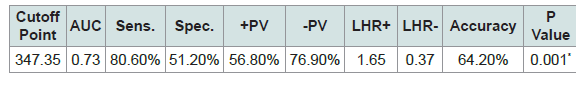
The best cutoff point of ACR to predict occurrence of preeclampsia is ≥347.35 mg/g with sensitivity of 80.6%, specificity of 51.2%, positive predictive value of 56.8% and negative predictive value of 76.9% (Table 4).
Table 4: Performance of Albumin Creatinine Ratio (ACR) as a diagnostic tool of preeclampsia among the studied groups.
Out of 70 females included in our study, 31 patients (55.7%) had significant proteinuria (>300 mg/day), while 62.9% of females had ACR greater than 347.35 mg/g while the remaining 37.1% showed their ACR level to be <347.35 mg/g.
Discussion
Prevalence of preeclampsia is 12% to 22% of all pregnancies. It has many risks on both the mother and the fetus with many adverse outcomes. So, obstetricians should be aware of any sign of this dangerous complication among women in the third trimester.
The most important step in diagnosis of preeclampsia is detection of albuminuria. In preeclampsia there is glomerular endothelial dysfunction which leads to defective glomerular basement membrane that leads to leakage of protein in urine
The quantity of protein loss has both diagnostic and prognostic implications, but what constitutes an ideal test or cut off values still remain controversial.
Three methods of urine protein detection have been used liberally in the current obstetric practice; urine dipstick analysis, the second one is 24 hours urinary proteins and the third one is the estimation of ratio of either protein or albumin to the creatinine concentration (urinary protein/creatine ratio (UPCR) and urine albumin/creatinine ratio (UACR)) in the random urine sample.
The dipstick method is easy to perform and cheap. However, it gives fluctuating results during the day related to changes in water intake, diet, exercise, postural changes or improper use of dipsticks. It is not a recommended test, as studies found significant false positive rates, poor sensitivity, and accuracy.
24 hour urine collection is the gold standard for detection of significant proteinuria in hypertensive women during pregnancy. However, it is time-consuming, subject to collection inaccuracy, requires good patient’s compliance and usually done on an in-patient basis. Also, if delivery was attempted before completion of 24 hour urine protein collection, there may be uncertain diagnosis of preeclampsia which may expose both mother and fetus to hazards. Also, it may increase health care costs and patient worries as there is increased hospital stay.
Urine albumin/creatinine ratio has many advantages; being a quantitative measure of protein which is not affected by different levels of urinary solutes or degree of dilution of urine. Also, being an accurate and rapid method of detection of proteinuria.
In this study, the main task was to evaluate the accuracy of albumin/creatinine ratio as a diagnostic method of proteinuria in pregnant patients with hypertensive disorders. This study included 70 pregnant women with hypertensive disorders admitted at the department of Obstetrics and Gynecology, Zagazig University Hospitals.
All included women had one form of hypertensive disorders with pregnancy (whether gestational hypertension, mild preeclampsia or severe preeclampsia). A systolic arterial blood pressure of 140 mmHg or more and a diastolic arterial blood pressure of 90 mmHg or more with or without proteinuria (detected by dipsticks) were settled as diagnostic criteria.
Our study showed that there were significant positive correlations detected between A/C ratio and 24 hour protein. While, there were significant negative correlations detected between A/C ratio and urine creatinine.
In this study we tried to get a means of quantifying proteinuria in a short period of time (spot urine Albumin/creatinine ratio). The best cutoff point of ACR to predict occurrence of preeclampsia was ≥347.35 mg/g with sensitivity of 80.6%, specificity of 51.2%, positive predictive value of 56.8% and negative predictive value of 76.9%.
The issue of reliability of measuring albumin/creatinine ratio in random urine samples as a substitute to the gold standard 24 hour urinary protein has been thoroughly studied over the last two decades; some authors showed a good correlation between them, while others found a poor correlation.
The International Society for the Study of Hypertension in Pregnancy (ISSHP) declared that random albumin/creatinine and total protein excretion in a 24 hour sample are equal in the diagnosis and classification of hypertensive disorders of pregnancy [7].
Nisell H et al. in 2006 and Rathindranath R et al. in 2015 suggested that in most cases the more cumbersome 24 hour urine collection can be replaced by the more convenient albumin/creatinine ratio on spot urine [8,9].
Amin SV et al. in 2014 showed that area under curve for urinary protein: creatine ratio (UPCR) was 0.89 (95% CI: 0.83 to 0.95), it had sensitivity of 82% and false positive rate of 12.5% for cutoff value of 0.45 (Figure 2) [10]. They used higher cutoff values (1.46 and 1.83) for prediction of heavy proteinuria (2 g and 3 g/24h, respectively). They concluded that random UPCR is a reliable investigation compared to dipstick method to assess proteinuria in hypertensive pregnant women. However, reference values should be standardized in clinical laboratories.
Cheung HC et al. in 2016 concluded that Spot urinary protein/creatine ratio had a positive and significant correlation with 24 hour urine results in Chinese preeclamptic women when the ratio was <200 mg/mmol [11]. Although, adverse pregnancy outcome was notpredicted by this ratio.
A recent metanalysis by Sanchez-Ramos L et al. in 2013 included twenty-four trials, published between January 1966 and April 2010 with 3,186 aggregate participants [12]. Accuracy of albumin/creatinine ratio was compared to 24 hour urine collection. Pooled sensitivities were 91.0% (95% CI 87.0-93.9), pooled specificities were 86.3% (95% CI 78.4-91.7), pooled positive likelihood ratio was 6.7 (95% CI 4.1, 10.9) and pooled negative likelihood ratio was 0.10 (95%CI 0.07, 0.16). Meta-regression analysis found that the studied co-variables did not affect test accuracy. In patients at risk for preeclampsia, random urine albumin/creatinine ratio is a useful evidence to exclude the presence of significant proteinuria. The best accuracy was found with a cut-off value of more than 0.30
Al RA et al. in 2004 studied 185 newly diagnosed hypertensive pregnant women and concluded that random urine protein to creatinine ratio was not a good predictor of significant proteinuria in such group of women [13].
Wikstrom AK et al. in 2006 did not recommend random A/C ratio for quantification of proteinuria in manifest preeclampsia, as A/C ratio may variate throughout the day and poorly correlated to protein estimation in 24 hour urine collection [14]. So, they recommended 24 hour urine collection, with measurement of total albumin or protein, when quantification of proteinuria is desired in women with preeclampsia with significant proteinuria.
Conclusion
ACR in random urine correlate well with 24 hour urine protein at a cutoff value of 347.35 mg/gm with sensitivity 80.6% and specificity 51.2% to detect protein excretion of 300 mg/24 hr, Therefore random ACR could be used as a rapid, easy and reliable test for diagnosis of significant proteinuria in hypertensive disorders with pregnancy, so it can substitute 24 hour urinary protein collection.
References
- Munir S (2013) Role of growth factors in preeclampsia: early detection and treatment. Avicenna 4: 1-9.
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, et al. (2010) Pregnancy hypertension. In: Williams obstetrics (23rdedn), McGraw-Hill's Access Medicine, New York, USA, pp. 706-756.
- Maynard SE, Thadhani R (2009) Pregnancy and the Kidney. J Am Soc Nephrol 20: 14-22.
- Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS (2007) Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol 196: 465.
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, et al. (2003) National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Inter Med 139: 137-147.
- Huang Q, Gao Y, Yu Y, Wang W, Wang S, et al. (2012) Urinary spot albumin: creatinine ratio for documenting proteinuria in Women with preeclampsia. Rev Obstet Gynecol 5: 9-15.
- Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM (2001) The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 20: IX–XIV.
- Nisell H, Trygg M, Back R (2006) Urine albumin/creatinine ratio for the assessment of albuminuria in pregnancy hypertension. Acta Obstet Gynecol Scand 85: 1327-1330.
- Rathindranath R, Teesta B, Proloy M (2015) Evaluation of spot urine protein/creatinine ratio versus 24 hour urine protein in diagnosis of hypertensive disorders of pregnancy. IOSR J Dent Med Sci 14: 44-47.
- Amin SV, Illipilla S, Hebbar S, Rai L, Kumar P, et al. (2014) Quantifying proteinuria in hypertensive disorders of pregnancy. Int J Hypertens 2014: 1-10.
- Cheung HC, Leung KY, Choi CH (2016) Diagnostic accuracy of spot urine protein-to-creatinine ratio for proteinuria and its association with adverse pregnancy outcomes in Chinese pregnant patients with pre-eclampsia. Hong Kong Med J 22: 249-255.
- Sanchez-Ramos L, Gillen G, Zamora J, Stenyakina A, Kaunitz AM (2013) The protein-to-creatinine ratio for the prediction of significant proteinuria in patients at risk for preeclampsia: a meta-analysis. Ann Clin Lab Sci 43: 211-220.
- Al RA, Baykal C, Karacay O, Geyik PO, Altun S, et al. (2004) Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol 104: 367-371.
- Wikström AK, Wikström J, Larsson A, Olovsson M (2006) Random albumin/creatinine ratio for quantification of proteinuria in manifest pre-eclampsia. BJOG 113: 930-934.