Journal of Andrology & Gynaecology
Download PDF
Research Article
Semen Quality of Male Partners of Infertile Couples Attending Fertility Clinics in Delta State University Teaching Hospital, Oghara, Delta State, Nigeria
Nanna Abimibola1* and Unuajohwofia Oghenevware2
- 1Department of Obstetrics/Gynaecology, Delta State University Teaching Hospital, Delta State, Nigeria
- 2Department of Surgery, Urology Division, Delta State University Teaching Hospital, Delta State, Nigeria
*Address for Correspondence: Nanna Abimibola, Department of Obstetrics/Gynaecology, Delta State University Teaching Hospital, Oghara, Delta State, Nigeria, Tel: 08053073834; E-mail: bolanannal@gmail.com
Citation: Nanna A, Unuajohwofia O. Semen Quality of Male Partners of Infertile Couples Attending Fertility Clinics in Delta State University Teaching Hospital, Oghara, Delta State, Nigeria. J Androl Gynaecol. 2017;5(1): 4.
Copyright: © 2017 Nanna A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Andrology & Gynaecology | ISSN: 2332-3442 | Volume: 5, Issue: 1
Submission: 26 April, 2017 | Accepted: 02 June, 2017 | Published: 12 June, 2017
Keywords
Semen analysis; Infertility; Teratozoospermia index
Abstract
Objective: To determine the proportion of sperm concentration abnormality, teratozoospermia index and the nature of semen abnormal parameters seen among male partners of infertile couples.
Materials and methods: This is a retrospective study of male partners of infertile couples who attended fertility clinic at Delta State University Teaching Hospital, Oghara, between 2014 - 2016. Semen samples of 260 male partners were analysed in the andrology laboratory using World Health Organization 2010 criteria for human semen characteristics.
Results: A total of 260 male partners were studied and 35% had primary infertility, 65% had secondary infertility and 53% had abnormal sperm concentration. The mean sperm concentration, morphology, progressive motility and teratozoospermia index for normazoospermia group are 72.7 ± 57.8, 4.2 ± 1.6, 61.1 ± 11.8, 1.7 ± 0.2 while the oligozoospermia group has 4.3 ± 4.1, 0.7 ± 1.1, 38.7 ± 7.5, 1.8 ± 0.4 at p - value 0.00. Azoospermia mean and SD for sperm concentration, morphology, progressive motility and teratzoospermia index are absent.
Conclusion: There is increased teratozoospermia index in normazoospermia while decreased mean normal sperm morphology and increased teratozoospermia index in oligozoospermia and these are absent in azoospermia subjects thereby causing male infertility which lead to hindrance in attaining pregnancy clinically.
Introduction
The inability of a couple to conceive after one year of unprotected sexual intercourse is term infertility [1]. The rate of infertility varies from one region to another and this corresponds to the incidence of preventable conditions which can lead to infertility [2].
In Sub-Saharan Africa, one third of the couples are infertile and 52% of this suffers from acquired infertility. Furthermore, clinical practice in Nigeria indicated that 50% of gynaecological consultations were due to infertility cases and 80% of laparoscopic investigations were for management of infertility [3]. Also, 8 - 12% of couples are affected with infertility in the world and 40 - 50% of cases of infertility are due to male factor [1,4]. In addition, males with secondary infertility are higher than males with primary infertility in Sub- Saharan Africa as indicated by World Fertility Survey [5].
In most cases, the aetiology of male infertility is largely unknown but studies have shown an increase trend in the prevalence of sexually transmitted infections and urogenital infections [6]. Male fertility has been affected with seminal tract infections through different mechanism which includes impairing spermatogenesis, sperm function and obstruction of the seminal tract [7]. Furthermore, endocrine disturbance, immunological condition, sexual dysfunction, varicocele and ejaculatory failures are other factors that lead to male infertility [8].
Male factor infertility can be assessed with the aid of semen analysis which form parts of the initial investigation undertaking by an infertile couple [5]. Semen analysis will reveal the abnormal semen parameters of male infertility which includes azoospermia, oligozoospermia, teratozoospermia and asthenozoospermia [9].
There has been a decline in semen quality in men according to recent reports and objectives of this retrospective study are [10]:
1. To determine the proportion of male partners of infertile couples with normal and abnormal sperm concentration.
2. To determine the nature of semen quality abnormality seen among males partners of infertile couples.
3. To determine the teratozoospermia index in infertile males.
Materials and Methods
This is a retrospective study of semen quality of male partners of infertile couples who presented at the fertility clinics of Delta State University Teaching Hospital, Oghara, between 2014 - 2016. Ethical approval was sought for from the Research and Ethics committee of the Hospital and it was given. Only males whose female partners were being investigated for infertility and with duration of 2 - 5 years of infertility were included in this study. Two hundred and sixty subjects submitted their seminal fluid for analysis after 3 day abstinence.
Semen samples were collected in a sterile universal plastic container by masturbation and the samples were delivered within one hour of collection. Semen analysis was carried out in the andrology laboratory of the Hospital using World Health Organization 2010 procedure to determine liquefaction, viscosity, volume, pН, sperm concentration, progressive motility, total motility, morphology and teratozoospermia index [11].
The World Health Organization 2010 normal values for semen parameters were use as operational definitions.
The data was analysed using SPSS version 15. Mean ± Standard deviation (SD) were calculated for sperm concentration, progressive motility, morphology, total motility volume, pН, Age, teratozoospermia index; 95% confidence interval was calculated for proportion and for means. Mean values were compared for statistical significance using t - value with level of significance < 0.05 (p - value). Correlations between variables were analysed using spearman’s rank correlation coefficient.
Results
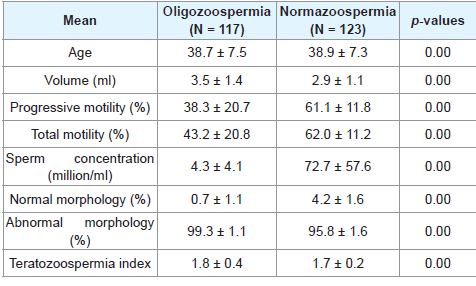
A total of 260 semen samples from male partners of infertile couples were analysed for retrospective study. The mean age of infertile male with azoospermia, oligozoospermia and normazoospermia are 41.4 ± 9.7, 38.7 ± 7.5, 38.9 ± 7.3 and infertility duration between 2 - 5 years.
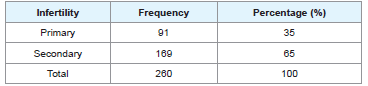
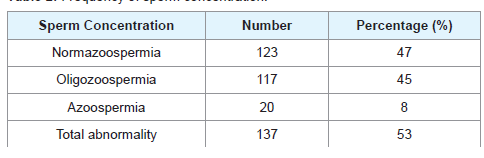
From Tables 1 and 2, 35% had primary infertility while 65% had secondary infertility. Sperm concentration characteristics indicated 47% normazospermia, 45% oligozoospermia and 8% azoospermia.
After excluding 20 samples for semen abnormality (Azoospermia), we analyzed oligozoospermic and normazoospermic males partners of infertile couples, as shown in Table 3.
In Table 3 below, there are abnormal mean sperm concentration, sperm morphology, increased teratozoospermia index and normal mean pН and volume and at significant p - value of 0.00 for the oligozooospermia groups while the normazoospermia group had normal mean semen parameter except increased teratozoospermia index. Furthermore, there is statistical significance between the mean semen parameters of normazoospermia and oligozoospermia group at a p - value of 0.00.
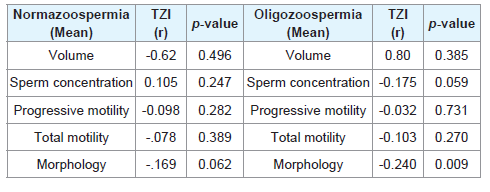
From Table 4, in the normazoospermia group, there is negative correlation between teratozoospermia index and, progressive motility, total motility, morphology, volume of semen but positive correlation with sperm concentration.
Table 4: Spearman’s rank correlation coefficient (r) and the corresponding p - value between continuous mean sperm variables and mean teratozoospermia index (TZI) of normazoospermia and oligozoospermia group.
In the oligozoospermia group, there is negative correlation between teratozoospermia index and sperm concentration, progressive motility, total motility, sperm morphology but positive correlation with semen volume. There is statistical significant negative correlation between TZI and sperm morphology, at a p - value of 0.009.
Discussion
There is positive association between abnormal semen parameters and sperm count. It has been shown that 90% of male infertility problems are as a result of sperm count and the problems of morphology and motility which stem from disarray in control mechanisms that include pre-testicular, testicular and post-testicular factors [12].
This study revealed that the prevalence of primary infertility was 35% while the prevalence of secondary infertility was 65%. This figure is higher than the reported values on the national estimates of the prevalence of primary and secondary infertility in sizeable areas of Sub-Saharan Africa [2]. This indicates the growing rate of secondary infertility in this area and could be attributed to high rate of genital infections in both male and female that leads to obstruction and male partners with abnormal semen parameters in our setting [13,14].
There was abnormal sperm concentration in 53% of male partners of infertile couples studied. This findings is in keeping with the earlier studies in Plateau State of Nigeria where 71% of semen samples analysed were abnormal and in agreement with similar high prevalence reported in India [15,16]. The abnormal sperm parameters with high prevalence in this study may contribute to higher infertility rate caused by male factors [17]. This may be as a result of sperm abnormalities which is usually associated with distortion in the process of spermatogenesis, be it pre-testicular (hormonal), testicular (chromosomal) or post-testicular (transportation disorder, ejaculation, infection) [18].
The mean sperm concentration for normazoospermia group was 72.7 ± 57.6 and 4.30 ± 4.1 for oligozoospermia group. This findings is comparable to work done in United Kingdom in which the mean sperm density was 84.3 ± 78.3 for 1801 suspected infertile men and 6.07 ± 5.6 for oligozoospermia patient in tertiary care hospital, Punjab [19,20]. From this study, 47% of male partners had normal sperm concentration while 45% had low count. This implies that the determinant of fertility of the male factor is not only sperm concentration but other factors like morphology, motility are equally important. Thus infertility is not only associated with decreased count but also with defective sperm parameters and other female factors [21].
The mean ejaculated volume for oligozoospermia group was 3.5 ± 1.4 vs. 2.9 ± 1.1 for normazoospermia group. This indicate that we had a normal volume for the two groups of male partners and this is comparable to studies done by Ara MJ et al. where normazoospermia had a mean semen volume of 3.36 ± 0.16 and oligozoospermia had 2.5 ± 0.17 [22]. The three days of sexual abstinence in our study may contribute to sufficient semen volume and our results indicated that semen volume plays little or no role in the aetiology of male infertility.
Sperm maturation is associated with motility as the sperm cells passed through the epididymis. The maturation of sperm cell occurred under the influence of epididymal protein and other substance which create structural and biochemical changes in the sperm. Thus motility is an epididymal function [23].
In this study, the mean percentage of progressive motility and total motility are 61.1% ± 11.8% and 62.6% ± 11.2% for normazoospermia while 38.3% ± 20.7% and 43.2% ± 20.8% for oligozoospermia. This is comparable to study done in the tertiary care hospital in Punjab where mean percentage of normal sperm motility were 38% ± 23% for oligozoospermia and 57% ± 0.18% for normazoospermia [20]. Other study done in Brazil indicated a mean progressive sperm motility of 36.9% ± 16% for normazoospermia [24].
Sperm motility has a stronger conception rate than sperm concentration [14]. This is a susceptible variation resulting from prolonged abstinence which is associated with increase sperm concentration while more frequent ejaculation may increase motility but lead to low sperm concentration [15].
In this study, the mean normal sperm morphology for normazoospermia is 4.2% ± 1.6% and 0.7% ± 1.1% for oligozoospermia. This is in support of the findings that relative levels of sperm morphology depend on the sperm concentration of the individual [20]. Furthermore, the normazoospermia morphology value is comparable to the study done for infertile males with normal sperm concentration in Brazil, where mean sperm morphology was 3.4% ± 2.9% and in the general population in Copenhagen area in Denmark where mean sperm morphology was 7.1% ± 4.9% [24,25].
Sperm morphology assessment is one of the most important steps in semen analysis for male partners of infertile couples [26]. It has been recorded in some studies that higher amount of abnormal sperm cells is associated with infertility [27]. This is confirmed in this study where higher amount of abnormal sperm cells for normazoospermia group (95.8% ± 1.6%) and oligozoospermia group (99.3% ± 1.1%) may be responsible for the infertility of the male partners in our setting.
In our study, the mean teratozoospermia index (TZI) for normazoospermia and oligozoospermia are 1.7 ± 0.2 and 1.8 ± 0.3 which are comparable with World Health Organization value of 1.81 ± 0.3 for male partners of infertile couples and 1.83 ± 0.57 for Pakistani infertile men [11,28]. Furthermore, TZI greater than 1.6 is associated with lower pregnancy rate and TZI of 1.81 is associated with male partners of infertile couples while TZI of 1.51 is associated with male partners of fertile couples [11,29].
Also, morphological parameters has been proven to be strongly associated with time in pregnancy and TZI or multiple anomalies index has been proven to be significantly related to the probability of a clinically recognised pregnancy [30].
Thus, in our study, normazoospermia male partners which accounts for 47% of the study population had normal mean sperm parameters but are still infertile. This could be as result of increased mean TZI which has negative correlation with mean sperm morphology and is associated with increased abnormal sperm cells (95.8% ± 1.6%). Also, oligozoospermia male partners which accounts for 45% of the study population had decreased normal mean sperm morphology and increased mean TZI which is a reflection of increased abnormal mean sperm morphology (99.3% ± 1.1%) and these could be responsible for the infertility in this group. Furthermore, this finding is supported by the fact that means normal sperm morphology lower than 5% in both studied population with increased abnormal forms, is associated with severe fertility problems [26]. Azoospermia male partners accounts for 8% of the study population which contribute to abnormal sperm concentration which leads to infertility in this group.
The increased abnormal sperm cells in this study is primarily an indication of the complexity of terminal sperm differentiation which involves several biochemical and morphological changes and the influence of micro or macro environmental factors or multiple genetic factors which modulate or disrupt the crucial stage of morphologenesis [31].
Conclusion
Semen analysis is an important clinical diagnosis step for infertile males and teratozoospermia index should be included as one of the parameters for semen quality and this will add value to clinical investigation for infertile males. Furthermore, research should be conducted in our environment to determine the causes of increase abnormal sperm cells which could be as a result of environmental or genetic factors.
Acknowledgement
We thank Dr. Patrick Okonta, Mrs. Kehinde Rachael and Mrs. Nonyelu Ifeoma Maureen for their contribution to this research work.
References
- World Health Organization Programme on Maternal and Child Health and Family Planning Unit (1991) Infertility: a tabulation of available data on prevalence of primary and secondary infertility. World Health Organization, Geneva, Switzerland.
- Larsen U (2000) Primary and secondary infertility in sub-Saharan Africa. Int J Epidemiol 29: 285-291.
- Orhue A, Aziken M (2008) Experience with a comprehensive university hospital-based infertility program in Nigeria. Int J Gynaecol Obstet 101: 11-15.
- Mehta RH, Makwana S, Ranga GM, Srinivasan RJ, Virk SS (2006) Prevalence of oligozoospermia and azoospermia in male partners of infertile couples from different parts of India. Asian J Androl 8: 89-93.
- Cates W, Farley TM, Rowe PJ (1985) Worldwide patterns of infertility: is Africa different? Lancet 2: 596-598.
- Ugboma HA, Obuna JA, Ugboma EW (2012) Pattern of seminal fluid analysis among infertile couples in a secondary health facility in South-Eastern Nigeria. Res Obstet Gynaecol 1: 15-18.
- Shaikh AH, Khalique K, Tariq G, Soomro N (2011) Pattern of semen abnormalities in couples with male factor infertility. Pak J Surg 27: 204-208.
- Monavari SH, Vaziri MS, Khalili M, Shamsi-Shahrabadi M, Keyvani H, et al. (2013) Asymptomatic seminal infection of herpes simplex virus: impact on male infertility. J Biomed Res 27: 56-61.
- Koster-Oyekan W (1999) Infertility among Yoruba women: perceptions on causes, treatments and consequences. Afr J Reprod Health 3: 13-26.
- Auger J, Kunstmann JM, Czyglik F, Jouannet P (1995) Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med 332: 281-285.
- World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen (5thedn). World Health Organization, Geneva, Switzerland, pp. 1-286.
- Iwamoto T, Nozawa S, Yoshiike M (2007) Semen quality of Asian men. Reprod Med Biol 6: 185-193.
- O EC, D EI, A BU, O C, U OI, et al. (2012) Male factor subfertility at the Imo State University Teaching Hospital, Orlu. J Gynaecol Obstet 16: 1-6.
- Adeniji RA, Olayemi O, Okunlola MA, Aimakhu CO (2003) Pattern of semen analysis of male partners of infertile couples at the University College Hospital, Ibadan. West Afr J Med 22: 243-245.
- Imade GE, Sagay AS, Pan LC, Ujah IO, Daru PH (2000) Seminal quality in male partners of infertile couples in Jos, Nigeria. Trop J Obstet Gynaecol 17: 24-26.
- Jajoo S, Kalyani KR (2013) Prevalence of abnormal semen analysis in patients of infertility at a rural setup in Central India. Int J Reprod Contracept obstet Gynecol 2: 161-164.
- Alemnji GA, Thomas KD (1997) Social-biological status of Nigerian males with primary and secondary infertility. East Afr Med J 74: 519-522.
- Owolabi AT, Fasubaa OB, Ogunniyi SO (2013) Semen quality of male partners of infertile couples in Ile-Ife, Nigeria. Niger J Clin Pract 16: 37-40.
- Mortimer D, Templeton AA, Lenton EA, Coleman RA (1982) Semen analysis parameters and their interrelationships in suspected infertile men. Arch Androl 8: 165-171.
- Butt F, Akram N (2013) Semen analysis parameters: experiences and insight into male infertility at a tertiary care hospital in Punjab. J Pak Med Assoc 63: 558-562.
- Peter AO, Temi AP (2016) Pattern of semen parameters and factors associated with infertility in males partners of infertile couples in Nigeria. Andrology (Los-Angel) 5: 162.
- Ara MJ, Hussain SM, Rashid MU (2015) Role of male partners in 100 infertile couples. JAFMC Bangladesh 11: 50-53.
- Akhtar MS, Akhtar FK (1991) Causes of male infertility. Pak J Med Res 30: 159-162.
- Borges E Jr, Setti AS, Braga DP, Figueira Rde C, Iaconelli A Jr (2015) Decline in semen quality among infertile men in Brazil during the past 10 years. Int Braz J Urol 41: 757-763.
- Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, et al. (2012) Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ open 2: e000990.
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, et al. (1988) Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 49: 112-117.
- Glatstein IZ, Harlow BL, Homstein MD (1998) Practice patterns among reproductive endocrinologists: further aspects of the infertility evaluation. Fertil Steril 70: 263-269.
- Ahmad MO, Inam-ul-haq, Kiyani MS, Zaheer M, Khan UA (2009) A study of teratozoospermia index in Pakistani men. Ann pak Inst Med Sci 5: 186-188.
- World Health Organization (1999) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction (4thedn). Cambridge University Press, UK, pp. 1-13.
- Slama R, Eustache F, Ducot B, Jensen TK, Jørgensen N, et al. (2002) Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod 17: 503-515.
- Auger J, Jouannet P, Eustache F (2016) Another look at human sperm morphology. Hum Reprod 31: 10-23.