Journal of Andrology & Gynaecology
Download PDF
Research Article
*Address for Correspondence: Vladimir Zaichick, PhD, DSc, Cchem, FRSC, Radionuclide Diagnostics Department, Medical Radiological Research Center, Koroleva Street 4, Obninsk, 249036, Kaluga Region, Russia, Tel: (48439) 60289; Fax: (495)956 1440; E-mail: vezai@obninsk.com
Citation: Zaichick V, Zaichick S. Variations in Concentration and Distribution of Several Androgen-Dependent and -Independent Trace Elements in Nonhyperplastic Prostate Gland Tissue throughout Adulthood. J Androl Gynaecol. 2016;4(1): 10.
Copyright © 2016 Zaichick V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Andrology & Gynaecology | ISSN : 2332-3442 | Volume: 4, Issue: 1
Submission: 27 January, 2016| Accepted: 01 March, 2016 | Published: 05 March, 2016
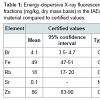
Table 1 presents the basic statistical parameters (arithmetic mean, standard deviation, standard error of mean, minimal and maximal values, median, percentiles with 0.025 and 0.975 levels) of the Br, Fe, Rb, Sr, and Zn concentration (mg/L) and the per cent volumes (% of volume) of the stroma, glandular epithelium, and glandular lumen in the nonhyperplastic prostate gland of males. These parameters are shown for the age groups 1 (range 21-40 years), 2 (range 41-60 years), 3 (range 61-87 years), in the age groups 2 and 3 combined (range 41-87 years), and in the age groups 1, 2, and 3 combined (range 21-87 years).Table 4 depicts our data for the inter-correlation of concentrations (values of r - the Pearson correlation coefficient) including all pairs of trace elements identified by us in age ranges 21-40 years and 41-87 years.
Variations in Concentration and Distribution of Several Androgen-Dependent and Independent Trace Elements in Nonhyperplastic Prostate Gland Tissue throughout Adulthood
Vladimir Zaichick1* and Sofia Zaichick1,2
- 1Radionuclide Diagnostics Department, Medical Radiological Research Center, Russia
- 2Department of Medicine, University of Illinois College of Medicine, Chicago, USA
*Address for Correspondence: Vladimir Zaichick, PhD, DSc, Cchem, FRSC, Radionuclide Diagnostics Department, Medical Radiological Research Center, Koroleva Street 4, Obninsk, 249036, Kaluga Region, Russia, Tel: (48439) 60289; Fax: (495)956 1440; E-mail: vezai@obninsk.com
Citation: Zaichick V, Zaichick S. Variations in Concentration and Distribution of Several Androgen-Dependent and -Independent Trace Elements in Nonhyperplastic Prostate Gland Tissue throughout Adulthood. J Androl Gynaecol. 2016;4(1): 10.
Copyright © 2016 Zaichick V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Andrology & Gynaecology | ISSN : 2332-3442 | Volume: 4, Issue: 1
Submission: 27 January, 2016| Accepted: 01 March, 2016 | Published: 05 March, 2016
Abstract
The variation with age of the Br, Fe, Rb, Sr, and Zn concentration in prostatic tissue and the relationship of these trace elements with basic histological structures of nonhyperplastic prostate glands of 65 subjects aged 21-87 years was investigated by an energy-dispersive X-ray fluorescence (EDXRF) and a quantitative morphometric analysis. Mean values ± standard error of the mean (M±SΕΜ) for the concentrations (mg/L) of these trace elements were: Br 7.89±0.93, Fe 22.2±1.2, Rb 3.49±0.18, Sr 0.43±0.08, and Zn 191±16. The significant trend for increase with age in Fe and Zn concentration as well as for increase with age in relative volume of stroma and decrease in relative volume of epithelium was found. Using the Pearson correlation between trace element concentration and morphometric parameters, it was demonstrated that the glandular lumen is a main pool of Sr and Zn accumulation in the normal human prostate, for the age range 21 to 87 years. It was concluded that the Sr and Zn bind tightly within the prostatic fluid, because the volume of glandular lumen reflects the volume of prostatic fluid. For ages above 40 years conclusive evidence of a disturbance in prostatic intracellular homeostasis of Fe was also shown.Keywords
EDXRF; Adult and geriatric prostate glands; Trace element concentrations; Trace element relationships; Trace element distributionsIntroduction
More than 70% the male population aged >60 years has clinical or histologic evidence of benign prostatic hyperplasia (BPH), while prostate cancer (PCa) is the most common male noncutaneous malignancy in the Western world [1,2]. Understanding etiologies of both conditions is crucial to reducing the resulting burden of mortality and morbidity.The prevalence of BPH rises sharply with age. The prevalence of PCa also drastically increases with age, being three orders of magnitude higher for the age group 40-79 years than for those younger than 40 years [3,4]. There are many similarities between the epidemiological factors of BPH and PCa but the greatest risk factor for both diseases is increasing age [5].
The human prostate gland is the only internal organ that continues to enlarge throughout adulthood [6,7]. Thus, it is possible to speculate that there are some age-dependence factors in prostate tissue which disturb a balance between normal cell proliferation and apoptosis. An elevated level of cell proliferation promotes BPH and PCa development. The etiology of both BPH and PCa is believed to be multifactorial. Both diseases may occur due to subtle changes in male hormones with age as well as other factors including levels of Ca, Zn, and other chemical elements in prostate tissue [8-14]. In our previous studies higher levels of Zn, Ca, and Mg as well as some other chemical elements were observed in prostate tissue of adult males when compared with nonprostatic soft tissues of the human body [15-19]. High accumulation of these elements suggests that they may play an important role in prostate function and health. Moreover, levels of some chemical elements were found to increase in the prostate tissue after puberty and throughout adulthood, and in some cases this increase was shown to be androgen-dependent [20-27]. The reason for this increase in chemical element content in the normal prostate gland is not completely understood. In addition, longstanding questions about the main pool and the local distribution of chemical elements in adult and geriatric prostate still remain open [28-37].
Prostatic tissue contains three main components: glandular tissue, prostatic fluid, and fibromuscular tissue or stroma. Glandular tissue includes acini and ducts. Epithelial cells (E) surround the periphery of the acini and luminal surfaces (L) in acini (glandular lumina). Prostatic fluid fills the lumina in the acini. Stromal tissue (S) is composed of smooth muscle, connective tissue, fibroblasts, nerves, lymphatic and blood vessels. Thus, the volume of the prostate gland may be represented as a sum of volumes (E+L+S). This makes it possible to quantitative morphological data using a stereological approach [20].
Cellular alterations that include changes in the epithelium and stroma are implicated in the development and growth of the prostate gland, as well as in BPH and PCa pathogenesis [38,39]. However, the data on age-dependence of main histological components of normal prostates is extremely limited [40,41]. Moreover, some contradictory results were obtained in these studies.
Because of the lack of adequate quantitative data on the subject of chemical element distributions in human prostate tissues and changes of these distributions with age, a study of as many of chemical elements as possible was begun by us. In our previous studies we investigated the chemical element distributions in pediatric and nonhyperplastic young adult prostate tissues using correlations between elemental contents and quantitative morphological data [20, 42-44]. It should be noted that the morphological data is assessed as % of volume, thus, the results for chemical element contents have to be expressed as a concentration on wet mass basis.
The primary purpose of present study was to determine reliable values for trace element concentrations and histological characteristics in the nonhyperplastic prostate of subjects ranging from young adult males to elderly persons (≥61 years old) using an energy-dispersive X-ray fluorescence (EDXRF) and a quantitative morphometric analysis. The second aim was to compare the trace element mass fractions and histological characteristics in prostate glands of age group 3 (elderly persons, who were aged 61 to 87 years), with those of group 1 (adults aged 21 to 40 years) and group 2 (adults aged 41 to 60 years). The third aim was to estimate the inter-correlations of trace element concentrations in normal prostate tissue. The final aim was to investigate the relationships between trace element concentrations in prostate tissue and quantitative morphometric parameters of the prostate glands studied. All studies were approved by the Ethical Committee of the Medical Radiological Research Center, Obninsk.
Materials and Methods
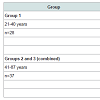
SamplesSamples of the human prostate were obtained from randomly selected autopsy specimens of 65 males (European-Caucasian) aged 21 to 87 years. Age ranges for subjects were divided into three age groups, with group 1, 21-40 years (30.4±1.1 years, M±SEM, n=28), group 2, 41-60 years (49.6±1.1 years, M±SEM, n=27), and group 3, 61-87 years (68.8±2.7 years, M±SEM, n=10). These groups were selected to reflect the condition of prostate tissue in the first (group 1) and in the second (group 2) period of adult life, as well as in the old age (group 3). The available clinical data were reviewed for each subject. None of the subjects had a history of an intersex condition, endocrine disorder, neoplasm or other chronic disease that could affect the normal development of the prostate. None of the subjects were receiving medications known to affect prostate morphology or its chemical element content. The typical causes of death of most of these patients included acute illness (cardiac insufficiency, stroke, pulmonary artery embolism, alcohol poisoning) and trauma. All prostate glands were divided (with an anterior-posterior crosssection) into two portions using a titanium scalpel. One tissue portion was reviewed by an anatomical pathologist while the other was used for the chemical element content determination. Only the posterior part of the prostate, including the transitional, central, and peripheral zones, was investigated. A histological examination was used to control the age norm conformity as well as to confirm the absence of any microadenomatosis and/or latent cancer.
Sample preparation
After the samples intended for the trace element determinations were weighed, they were transferred to be stored at -20 °C, until they were freeze-dried, weighed once again and homogenized. The pounded sample weighing about 8 mg was applied to a piece of adhesive tape, which served as a sample backing. Titanium or plastic tools were used in sampling and sample preparation for the trace element determinations [45-47].
The prostate specimens intended for the morphometric study were transversely cut into consecutive slices, which were fixed in buffered formalin (pH 7.4) and embedded in paraffin wax. The paraffin-embedded specimens were sectioned with 5 μm thickness and processed using routine histological methods. All samples were conventionally stained with haematoxylin and eosin, and then all histological slides were examined by an anatomical pathologist to detect any focus of benign prostatic hyperplasia, carcinoma, or intraepithelial neoplasia, to exclude samples with artifacts and so to select appropriate slides for further morphometric evaluation.
Standards and certified reference materials
To determine concentration of the elements by comparison with known standard, aliquots of commercial, chemically pure compounds were used for a calibration [48]. Ten certified reference materials (CRM) IAEA H-4 (Animal Muscle) sub-samples were prepared and analyzed under the same conditions as the prostate samples, to estimate the precision and accuracy of the results. All samples of prostate tissue and the CRM were prepared in duplicate and mean values of Br, Fe, Rb, Sr, and Zn concentrations were used in the final calculation.
Instrumentation and methods
The facility for EDXRF analysis included an annular 109Cd source with an activity of 2.56 GBq, a Si(Li) detector and a PC based portable multichannel analyzer. Its resolution was 270 eV at the 5.9 keV line of a 55Fe-source. The duration of the trace elements measurements was 60 min. The intensity of the Kα-line of Br, Fe, Rb, Sr, and Zn for both samples and standards was estimated using a calculation based on the total area under the corresponding photopeak in the spectra. The trace element concentration was calculated by the relative method of comparing between intensities of theKα-lines for samples and standards.
Morphometric evaluations were then performed quantitatively using stereological method [49]. The stained tissue sections were viewed by microscopy at ×120 magnification. In order to obtain information about changes in prostatic components (acini and stroma), the surfaces adjacent to the acini (i.e. epithelium plus lumen), the epithelium tissue alone and the stroma were also measured in 10 randomly selected microscopic fields for each histological section. The number of microscopic fields per section studied was determined by successive approaches to obtain the minimum number of microscopic fields required to reach the lowest standard deviation (SD). A greater number of microscopic fields did not decrease the SD significantly. The mean per cent volumes of the stroma, glandular epithelium, and glandular lumen were determined for each prostate specimen.
Computer programs and statistic
Using Microsoft Office Excel software to provide a summary of statistical results, the arithmetic mean, standard deviation, standard error of mean, minimum and maximum values, median, percentiles with 0.025 and 0.975 levels were calculated for all the trace element concentrations obtained as well as for the morphometric parameters. The reliability of difference in the results between all age groups was evaluated by Student’s parametric t-test. The Microsoft Office Excel software was also used for the construction of “trace element concentration versus age”, “morphometric parameter versus age”, and “trace element concentration versus morphometric parameter” diagrams and the estimation of the Pearson correlation coefficient between the different pairs of trace elements as well as between the morphometric parameters and trace element concentrations.
Results
Figure 1 illustrates individual data sets for the Br, Fe, Rb, Sr, and Zn concentration and the per cent volume (stroma, epithelium, and lumen) in the nonhyperplastic prostate gland of males aged between 21-87 years and their trend lines with equations of best fit.Figure 1: Individual data sets for the Br, Fe, Rb, Sr, and Zn concentrations (mg/L) and the percent volume of stroma, epithelium, and lumen in the nonhyperplastic prostate gland of males aged 21-87 years, plotted against age, with the corresponding trend lines and the equations from which they were derived.
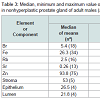
The comparison of our results with published data for the Br, Fe, Rb, Sr, and Zn concentrations [50-58] and for the morphometric parameters of the nonhyperplastic prostate gland of adult males [40,41] is shown in Table 2.
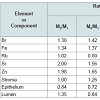
The ratios of means and the reliability of difference between mean values of trace element concentrations and between mean values of morphometric parameters in the age groups 1, 2, 3, and (2 and 3) combined are presented in Table 3.
Table 3: Median, minimum and maximum value of means of chemical element concentrations (mg/L) and the per cent volumes of main histological components (%) in nonhyperplastic prostate gland of adult males (age range ≥ 21 years) according to data from the literature in comparison with this work’s results.
Table 5 compiles Pearson correlation coefficients between the Br, Fe, Rb, Sr, and Zn concentrations (mg/L) and the morphometric parameters (% of volume) in age range 21-40 years and 41-87 years. Figure 2 shows individual data sets for the Fe concentration versus individual data sets for the percent volume of stroma and epithelium in the nonhyperplastic prostate gland of males between ages 21-40 years and 41-87 years.
Discussion
Precision and accuracyA set of existing international CRMs prepared from the soft tissues of humans and animals is extremely limited. As was previously discussed 97% of the self-absorption in the dry sample of prostate is due to the content of bulk elements (C, N, O, P, S) and main electrolytes (Ca, Cl, Na) [58]. The content of these elements and the mass density of muscle and prostate in humans are virtually identical [59,60]. It is not surprising because smooth muscles comprise 25-30% of the total normal prostate gland tissue [38,39]. There is no reason to believe that the content of the above-mentioned elements in the muscles of animals is significantly different from their content in human muscles. Therefore, the use of CRM IAEA H-4 as a CRM for the EDXRF analysis of samples of prostate tissue is acceptable.
We determined the mass fractions of five trace elements (Br, Fe, Rb, Sr, and Zn) that include the range of 4 elements (Br, Fe, Rb, and Zn) available in the CRM and one element (Sr) with informative values in the CRM IAEA H-4 (animal muscle). Mean values (±SD) for Br, Fe, Rb, and Zn (Table 1) were inside the 95% confidence intervals of the values listed on the CRM’s certificate. Good agreement of the Br, Fe, Rb, Sr, and Zn mass fractions analyzed by EDXRF with the certified data of CRM IAEA H-4 indicates an acceptable accuracy for the results obtained in this study of trace elements in the prostate, presented in Figures 1 and 2 and Tables 2-5.
Concentration of trace elements
Table 2 summarizes mean values and all selected statistical parameters were calculated for five trace elements (Br, Fe, Rb, Sr, and Zn). Concentrations of all these elements were measured in major portion of prostate samples. Since we were using EDXRF the results were expressed as mass fractions (MF) in mg/kg on dry mass basis, and the concentration Cij for the i element in the j sample was calculated as:
Cij (mg/L) = MFij × (Mj dry/Mjwet) × 1.05 [1]
Where Mjdry is the mass of j sample after drying, Mjwet is the mass of j sample before drying, and 1.05 (kg/L) is the density of normal prostate tissue [59].
Comparison with published data
The values of arithmetic mean obtained for the Br, Fe, Rb, Sr, and Zn concentrations in adult nonhyperplastic prostate glands (Table 3) agree well with median of means cited by other researches for the normal prostate tissue of adult males (age range ≥ 21 years), including samples obtained from persons who had died from non-prostate related diseases. A number of previously published values for chemical element contents were not expressed as concentration by the authors of the cited references. We recalculated these values using published data for water -83% [61] and ash - 1.0% [62] content on a wet-mass basis for the prostates of adult men as well as data for adult prostate tissue density -1.05 kg/L [59]. The means of morphometric parameters for adult nonhyperplastic prostate glands found in the present study also agree well with median of means cited by other researches (Table 3).
Age-related changes
The similarity of arithmetic mean and median values for all the parameters investigated (Table 2) testifies to the normal distribution of individual results. These findings allowed evaluation of the age-related differences by Student’s parametric t-test (Table 4). In the histological normal prostates of adults we observed a statistically significant increase with age of Fe and Zn concentrations as well as in per cent volume of stroma and lumen, accompanied by a decrease in per cent volume of lumen (Figure 1 and Table 4). The conclusion from the analysis of individual data sets obtained from the histologically normal prostates (Figure 1) and from the comparison of the concentration means in three age groups (Table 4) is that concentration of Fe and Zn increased in the third to sixth decades and reached a maximum at about the age of 50-60 years. After age 60 years, levels of Fe and Zn were maintained at more or less steady levels (Figure 1 and Table 4). Therefore, main changes in these prostate tissue trace element levels occur between ages 21 and 60 years. We can also conclude that the trace element concentration in the geriatric prostate does not differ from that of the prostate gland of 41 to 60 year old adult males.
In accordance with earlier findings, we also found that Zn concentration is age-dependent [63-67]. For example, Heinzsch et al. found that Zn content in normal prostate was higher in the age group 51-70 years than in the age group 31-50 years by approximately 1.8 times [63]. In accord with results of Tohno et al. there were no significant correlations between ages and the Fe or Zn content in prostate tissue of Thai subjects, who ranged in age from 43 to 86 years and of Japanese subjects ranging in age from 65 to 101 years [67].
In the histological normal adult prostates mean percent volumes of stroma was maintained at about 50% and only increased above this value in the seventh decade (Figure 1 and Table 2). In the group older than 70 years old stroma volume increased ~1.3 times (60.8%, age group 3), which was statistically significant (Tables 2 and 4). The mean percent volume of the glandular epithelium steadily and almost linearly decreased from 35.7% to 28.4% over the same period (Figure 1 and Table 2). These differences were statistically significant for the age group 3 when compared with the age groups 1 or 2 (Table 4). The mean percent volume of the glandular lumen increased between the third to the fifth decade and reached its maximum at about 50 years old (Figure 1). During this period of life the mean per cent volume of glandular lumen was almost 1.5 times higher than in prostate glands of 20 to 30 year old males, which is statistically significant (Table 4). This suggests that relative accumulation of prostatic fluid develops between 30 to 50 years of age.
Shapiro et al. reported that the stromal compartment fraction (approximately 80%) of the prostate remains constant in males throughout life [68]. In contrast, the present study provides compelling evidence that the per cent volume of stroma, epithelium, and lumen of the prostate changes significantly in males between ages 21-70. Our finding is in agreement with an earlier publication by Arenas et al where he reported that the stromal volume was maintained between ages 20-50 and only significantly increased in the sixth and seventh decades, while epithelial volume showed a tendency to diminish [40].
Inter-correlations of trace elements
In the age group 1 (21-40 years) a statistically significant (p≤0.05) direct correlation was found between the prostatic concentration of Zn and Rb (r=0.38), Fe and Rb (r=0.35), Fe and Sr (r=0.30), Br and Rb (r=0.33), and also between Br and Sr (r=0.27) (Table 5). In age groups 2 and 3 taken together (41-87 years) many correlations between trace elements in the prostate, found in the age group 1 (21-40 years), disappeared (Table 5). Therefore, if we accept levels and relationships of trace elements in prostate glands of 21-40 year old males as a norm, then we have to conclude that after the age of 40 there are significant changes in levels and balance of trace elements in the prostate.
Correlations between trace element concentrations and the per cent volumes of main histological components
A significant positive correlation between the prostatic Zn concentration and per cent volume of the glandular lumen (r=0.507, p≤0.01) as well as between the Sr concentration and per cent volume of glandular lumen (r=0.393, p≤0.05) was seen in the age group 1 (Table 6). These correlations were also found in age groups of males aged above 40 (group 2 and 3 combined). This indicates that there is a special relationship between Zn and the glandular lumen of the prostate. In other words, the glandular lumen is a main pool of Zn accumulation in the normal human prostate. Zn concentration in prostatic fluid is approximately five times higher than the mean Zn concentration in prostate tissue of adult males [20]. Because the volume of the glandular lumen reflects the volume of prostatic fluid, we can conclude that Zn concentration in prostatic fluid is higher than in prostatic cells throughout adulthood. In addition to Zn, Ca concentration in prostatic fluid is also a few times higher than the mean Ca concentration in the prostate tissue of adults [42,43]. Therefore, Ca concentration in prostatic fluid is also higher than in prostatic cells. Since Ca and Sr belong to the same group of the Periodic Table we can extrapolate and state there is also a significant positive correlation between the prostatic Sr concentration and the per cent volume of the glandular lumen throughout adulthood.
A pronounced positive correlation between the prostatic Fe concentration and the per cent volume of stroma (r=0.369, p≤0.05) accompanied by a negative correlation between Fe concentration and the per cent volume of epithelium (r=-0.425, p≤0.05) was also observed in the age group 1 (Table 6). In age groups of males aged above 40 (group 2 and 3 combined) these correlations also vanished (Figure 2 and Table 6). It is well known that blood is the main pool of Fe in the human body [59]. Fe concentration in the adult whole blood is about 500 mg/L or 20 times higher than in the prostate tissue (Table 2) [69]. This indicates that prostatic cells and fluid contain much less Fe compared to blood. Fe concentration in blood does not increase with age. Therefore, it is possible to speculate that in the age range 21 to 40, when the per cent volume of stroma is more or less stable, individual Fe concentration variations reflect individual levels of stromal vascularization. We showed that in age groups above 40 there is a rapid increase in the stromal volume. Consequently, this increase may upset the balance between vascularization and intracellular homeostasis of Fe in prostate tissue.
The role of Zn and Fe excess in an age-related enlargement and malignancy of the prostate
Adult mean Zn concentration in the prostate increase from 127 mg/L to 210 mg/L in the sixth decade. This level of the prostatic Zn concentration is higher than a mean value of its content in all other tissues (soft and hard) of the human body including skeletal muscle, liver, lung, kidney, and bones [59,60, 69]. Excessive Zn levels may be harmful to normal metabolism of cells and partially responsible for the age-related enlargement of the prostate and its malignant transformation. There are multiple reasons which imply that the age-related excessive Zn levels in prostatic tissue is probably one of the main factors influencing the enlargement of prostate and the development of PCa in stages of initiation and promotion. This was discussed in details in our previous publications [20,25,27].
Despite the fact that Fe is an essential element, it is also potentially toxic in excess because free Fe ions inside the cell can lead to the generation of free radicals that cause oxidative stress and cellular damage [70-73]. It was shown that the mean prostatic Fe concentration increase from 18.7 mg/L to 25.5 mg/L in the sixth decade. Therefore, it is reasonable to speculate that similar to elevated Zn levels, excessive levels of Fe in prostatic tissue and disturbance in intracellular homeostasis of Fe with age are probably two of the factors influencing benign enlargement and malignant transformation of the prostate.
The limitations
To clarify the role of trace elements in normal physiology of the prostate gland, the variation with age of the Br, Fe, Rb, Sr, and Zn concentration in prostatic tissue and the relationship of these trace element concentrations with basic prostatic histological structures was investigated only in nonhyperplastic prostate glands. In future studies of the role of trace elements in pathophysiology of the prostate gland the specimens of BPH and cancerous tissues have to be included. Moreover, there are many other chemical elements involved in normal metabolism and pathophysiology of the prostate gland. Thus, further studies are needed to extend the list of chemical elements investigated in this manner.
Conclusion
While the numbers of specimens were somewhat limited, they were sufficient to identify the Fe and Zn concentration differences in the three age groups studied. The Pearson correlation between trace element concentrations and morphometric parameters allowed allocation of trace element concentrations to the different components of the prostate gland. Using this method, we demonstrated that the glandular lumen and, therefore, the prostatic fluid is the main pool of Sr and Zn accumulation in the normal human prostate between the ages of 21 to 87. We also found that the stroma is the main pool of Fe accumulation in the normal human prostate, which correlates with the level of prostate tissue vascularization. Lastly, we found that there is a significant tendency for an increase in Fe and Zn concentration with age in the prostate tissue of healthy individuals. All these factors are very likely to contribute to the age-related benign enlargement and potentiate malignant transformation of the prostate.Acknowledgements
The authors are grateful to the late Prof. A.A. Zhavoronkov, Institute of Human Morphology, Russian Academy of Medical Sciences, Moscow, for supplying prostate specimens and for his help in the morphometric study. The authors are also grateful to Dr. Sinclair Wynchank for a very valuable and detailed discussion of the results of this work.References
- Roehrborn CG, McConnell J (2002) Etiology, pathophysiology, epidemiology and natural history of benign prostatic hyperplasia. In: Walsh P, Retik A, Vaughan E, Wein A (Eds) Campbell’s Urology (8th Edn), Saunders, Philadelphia, pp. 1297-1336.
- Velonas VM, Woo HH, dos Remedios CG, Assinder SJ (2013) Current status of biomarkers for prostate cancer. Int J Mol Sci 14: 11034-11060.
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, et al. (2003) Cancer statistics, 2003. CA Cancer J Clin 53: 5-26.
- Rebbeck TR (2006) Conquering cancer disparities: new opportunities for cancer epidemiology, biomarker, and prevention research. Cancer Epidemiol Biomarkers Prev 15: 1569-1571.
- Alcaraz A, Hammerer P, Tubaro A, Schröder FH, Castro R (2009) Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur Urol 55: 864-873.
- Bonkhoff H, Remberger K (1998) Morphogenesis of benign prostatic hyperplasia and prostatic carcinoma. Pathologe 19: 12-20.
- Schauer IG, Rowley DR (2011) The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation 82: 200-210.
- Thomas JA (1999) Diet, micronutrients and the prostate gland. Nutr Rev 4: 95-103.
- Zaichick V, Zaichick S, Friedrich-Schiller Universitat (1999) Role of zinc in prostate cancerogenesis. In: Stock and trace elements, mineral and trace elements, Verlag Harald Schubert, Leipzig, Germany, pp. 104-115.
- Blumenfeld AJ, Fleshner N, Casselman B, Trachtenberg J (2000) Nutritional aspects of prostate cancer: a review. Can J Urol 7: 927-935.
- Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, et al. (2003) Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst 95: 1004-1007.
- Zaichick V (2004) INAA and EDXRF applications in the age dynamics assessment of Zn content and distribution in the normal human prostate. J Radioanal Nucl Chem 262: 229-234.
- Ahn J, Albanes D, Peters U, Schatzkin A, Lim U, et al. (2007) Dairy products, calcium intake, and risk of prostate cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 16: 2623-2630.
- Rowland GW, Schwartz GG, John EM, Ingles SA (2013) Protective effects of low calcium intake and low calcium absorption vitamin D receptor genotype in the California Collaborative Prostate Cancer Study. Cancer Epidemiol Biomarkers Prev 22: 16-24.
- Zaichick S, Zaichick V, Karandashev V, Ermidou-Pollet S, Pollet S (2010) The effect of age and gender on the lithium content in rib bone of healthy humans. Trace Elem Electrolytes 27: 258-261.
- Zaichick S, Zaichick V (2011) INAA application in the age dynamics assessment of Br, Ca, Cl, K, Mg, Mn, and Na content in the normal human prostate. J Radioanal Nucl Chem 288: 197-202.
- Zaichick S, Zaichick V (2011) The effect of age on Ag, Co, Cr, Fe, Hg, Sb, Sc, Se, and Zn contents in intact human prostate investigated by neutron activation analysis. Appl Radiat Isot 69: 827-833.
- Zaichick S, Zaichick V, Nosenko S, Moskvina I (2012) Mass fractions of 52 trace elements and zinc/trace element content ratios in intact human prostates investigated by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 149: 171-183.
- Zaichick V, Nosenko S, Moskvina I (2012) The effect of age on 12 chemical element contents in intact prostate of adult men investigated by inductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res 147: 49-58.
- Zaichick S, Zaichick V (2013) Relations of morphometric parameters to zinc content in paediatric and nonhyperplastic young adult prostate glands. Andrology 1: 139-146.
- Zaichick V, Zaichick S (2013) The effect of age on Br, Ca, Cl, K, Mg, Mn, and Na mass fraction in pediatric and young adult prostate glands investigated by neutron activation analysis. Appl Radiat Isot 82: 145-151.
- Zaichick V, Zaichick S (2013) INAA application in the assessment of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn mass fraction in pediatric and young adult prostate glands. J Radioanal Nucl Chem 298: 1559-1566.
- Zaichick V, Zaichick S (2013) NAA-SLR and ICP-AES application in the assessment of mass fraction of 19 chemical elements in pediatric and young adult prostate glands. Biol Trace Elem Res 156: 357-366.
- Zaichick V, Zaichick S (2013) Use of neutron activation analysis and inductively coupled plasma mass spectrometry for the determination of trace elements in pediatric and young adult prostate. Am J Analyt Chem 4: 696-706.
- Zaichick V, Zaichick S (2014) INAA application in the assessment of chemical element mass fractions in adult and geriatric prostate glands. Appl Radiat Isot 90: 62-73.
- Zaichick V, Zaichick S (2014) Determination of trace elements in adults and geriatric prostate combining neutron activation with inductively coupled plasma atomic emission spectrometry. Open J Biochem 1: 16-33.
- Zaichick V, Zaichick S (2014) Use of INAA and ICP-MS for the assessment of trace element mass fractions in adult and geriatric prostate. J Radioanal Nucl Chem 301: 383-397.
- Mawson CA, Fischer MI (1952) The occurrence of zinc in the human prostate gland. Can J Med Sci 30: 336-339.
- Delory GE, Hoare R, Penner DW (1956) Zinc and acid phosphatase in the human prostate. Cancer 9: 721-726.
- Siegal E, Graig FA, Crystal MM, Siegal EP (1961) Distribution of 65Zn in the prostate and other organs of man. Br J Cancer 15: 647-664.
- Kar AB, Chowdhury AR (1968) Distribution of zinc in the subcellular fractions of human prostate. Curr Sci 37: 375-376.
- Dhar NK, Goel TC, Dube PC, Chowdhury AR, Kar AB (1973) Distribution and concentration of zinc in the subcellular fractions of benign hyperplastic and malignant neoplastic human prostate. Exp Mol Pathol 19: 139-142.
- Morita H (1981) Histochemical study of human prostate. Ultrastructural and XMA observation of zinc in the hyperplastic and neoplastic prostate (author’s transl). Jap J Urol 72: 717-729.
- Leake A, Chisholm GD, Habib FK (1983) The distribution of zinc in human prostate. Prostate 4: 421-422.
- Tvedt KE, Halgunset J, Kopstad G, Haugen OA (1989) Intracellular distribution of calcium and zinc in normal, hyperplastic, and neoplastic human prostate: X-ray microanalysis of freeze-dried cryosections. Prostate 15: 41-51.
- Bataineh ZM, Bani Hani IH, Al-Alami JR (2002) Zinc in normal and pathological human prostate gland. Saudi Med J 23: 218-220.
- Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, et al. (2005) hZIPI zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 4: 32.
- Chagas MA, Babinski MA, Costa WS, Sampaio FJ (2002) Stromal and acinar components of the transition zone in normal and hyperplastic human prostate. BJU Int 89: 699-702.
- Zhang Y, Nojima S, Nakayama H, Jin Y, Enza H (2003) Characteristics of normal stromal components and their correlation with cancer occurrence in human prostate. Oncol Rep 10: 207-211.
- Arenas MI, Romo E, Royuela M, Ruiz A, Fraile B, et al. (2001) Morphometric evaluation of the human prostate. Int J Androl 24: 37-47.
- Fujikawa S, Matsuura H, Kanai M, Fumino M, Ishii K, et al. (2005) Natural history of human prostate gland: Morphometric and histopathological analysis of Japanese men. Prostate 65: 355-364.
- Zaichick V, Zaichick S (2014) Relations of the neutron activation analysis data to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. Adv Biomed Sci Eng 1: 26-42.
- Zaichick V, Zaichick S (2014) Relations of the Al, B, Ba, Br, Ca, Cl, Cu, Fe, K, Li, Mg, Mn, Na, P, S, Si, Sr, and Zn mass fractions to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. Biometals 27: 333-348.
- Zaichick V, Zaichick S (2014) The distribution of 54 trace elements including zinc in pediatric and nonhyperplastic young adult prostate gland tissues. J Clin Lab Investig Updates 2: 1-15.
- Zajchik VE, Zajchik SV (1996) Instrumental effect on the contamination of biomedical samples in the process of sampling. J Analyt Chem 51: 1322-1327.
- Zaichick V (1997) Sampling, sample storage and preparation of biomaterials for INAA in clinical medicine, occupational and environmental health. In: Harmonization of health-related environmental measurements using nuclear and isotopic techniques, International Atomic Energy Agency, Vienna, pp. 123-133.
- Zaichick VY (2006) Medical elementology as a new scientific discipline. J Radioanal Nucl Chem. 269: 303-309.
- Zaichick V (1995) Application of synthetic reference materials in the medical radiological research Centre. Fresenius J Anal Chem 352: 219-223.
- Avtandilov GG (1973) Morphometry in pathology. Medicina , Moscow.
- Kubo H, Hashimoto S, Ishibashi A (1976) Simultaneous determinations of Fe, Cu, Zn, and Br concentrations in human tissue sections. Med Phys 3: 204-209.
- Sangen H (1967) The influence of the trace metals upon the aconitase activity in human prostate glands. Jap J Urol 58: 1146-1159.
- Jafa A, Mahendra NM, Chowdhury AR, Kamboj VP (1980) Trace elements in prostatic tissue and plasma in prostatic diseases of man. Indian J Cancer 17: 34-37.
- Stitch SR (1957) Trace elements in human tissue. 1. A semi-quantitative spectrographic survey. Biochem J 67: 97-103.
- Soman SD, Joseph KT, Raut SJ, Mulay GD, Parameswaran M, et al. (1970) Studies of major and trace element content in human tissues. Health Phys 19: 641-656.
- Tipton IH, Isabel H, Cook MJ (1963) Trace elements in human tissue Part II. Adult subjects from the United States. Health Phys 9: 103-145.
- Zaichick S, Zaichick V (2011) The Br, Fe, Rb, Sr, and Zn content and interrelation in intact and morphologic normal prostate tissue of adult men investigated by energy-dispersive X-ray fluorescent analysis. Xray Spectrom 40: 464-469.
- Galván-Bobadilla AI, García–Escamilla RM, Gutiérrez-García N, Mendoza-Magaña ML, Rosiles-Martínez R (2005) Cadmium and zinc concentrations in prostate cancer and benign prostatic. Lat Am J Clin Pathol Lab Med 52: 109-117.
- Zaichick S, Zaichick V (2010) Method and portable facility for energy-dispersive X-ray fluorescent analysis of zinc content in needle-biopsy specimens of prostate. Xray Spectrom 39: 83-89.
- International Commission on Radiological Protection (1975) Report of the task Group on reference Man: ICRP Publication 23, Pergamon Press, Oxford.
- Iyengar GV, Kollmer WE, Bowen HJ (1978) The elemental composition of human tissues and body fluids: a compilation of values for adults. Verlag Chemie, Weinheim, New York.
- Marezynska A, Kulpa J, Lenko J (1983) The concentration of zinc in relation to fundamental elements in the diseased human prostate. Int Urol Nephrol 15: 257-265.
- Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS (1990) Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environ Res 52: 126-145.
- Hienzsch E, Schneider HJ, Anke M (1970) Vergleichende Untersuchungen zum Mengen- und Spurenelementgehalt der normalen Prostata, des Prostataadenoms und des Prostatakarzinoms. Z Urol Nephrol 63: 543-546.
- Leissner KM, Fielkegard B, Tisell LE (1980) Concentration and content of zinc in human prostate. Invest Urol 18: 32-35.
- Tisell L, Fielkegard B, Leissner KM (1982) Zinc concentration and content of the dorsal, lateral and medial prostatic lobes and of periurethral adenomas in man. J Urol 128: 403-405.
- Oldereid NB, Thomassen Y, Attramadal A, Olaisen B, Purvis K (1993) Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J Reprod Fertil 99: 421-425.
- Tohno S, Kobayashi M, Shimizu H, Tohno Y, Suwannahoy P, et al. (2009) Age-related changes of the concentrations of select elements in the prostates of Japanese. Biol Trace Elem Res 127: 211-227.
- Shapiro E, Hartanto V, Perlman EJ, Tang R, Wang B, et al. (1997) Morphometric analysis of pediatric and nonhyperplastic prostate glands: Evidence that BPH is not a unique stromal process. Prostate 33: 177-182.
- Iyengar GV (1998) Reevaluation of the trace element content in reference men. Radiat Phys Chem 51: 545-560.
- Weinberg ED (1992) Roles of iron in neoplasia. Promotion, prevention, and therapy. Biol Trace Elem Res 34: 123-140.
- Toyokuni S (2009) Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci 100: 9-16.
- Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283: 65-87.
- Torti SV, Torti FM (2013) Iron and cancer: more ore to be mined. Nat Rev Cancer 13: 342-355.