Advances in Diabetes & Endocrinology
Download PDF
Research Article
Clinician’s Perspectives on the use of Vildagliptin and its Fixed-Dose Combination with Metformin in the Management of Type 2 Diabetes Mellitus in India
Manjula S* and Krishna Kumar M
Department of Medical Services, Micro Labs Limited, Bangalore, Karnataka, India
*Address for Correspondence:Dr Manjula S, Department of Medical Services, Micro Labs
Limited, Bangalore, Karnataka, India. E-mail Id: drmanjulas@gmail.com
Submission: 20 May, 2025
Accepted: 12 June, 2025
Published: 14 June, 2025
Copyright: © 2025 Manjula S, et al. This is an open access
article distributed under the Creative Commons Attribution
License, which permits unrestricted use, distribution, and
reproduction in any medium, provided the original work is
properly cited.
Keywords:Type 2 Diabetes; Vildagliptin, Metformin; Middle-aged
Patients; Medication Adherence; Glycemic Control
Abstract
Objective: To gather clinician perspectives related to the initiation
and management of pharmacotherapy in type 2 diabetes mellitus
(T2DM), with a particular focus on the use of vildagliptin and its fixed dose
combination (FDC) with metformin in Indian clinical settings.
Methods: This cross-sectional study was conducted among clinicians across India. The 23-item questionnaire explored various aspects of T2DM management, including demographic patterns, first-line therapy preferences, treatment adherence, perceptions of glycemic durability, and clinical experiences with vildagliptin and its sustained-release formulations. Additional questions addressed socioeconomic trends in diabetes prevalence, factors contributing to nonadherence, and the role of continuous glucose monitoring. Data were analyzed using descriptive statistics.
Results: A total of 242 clinicians participated in the study. The majority (82.64%) identified the highest prevalence of diabetes in the 40–60-year age group, and 71% reported a higher occurrence among middle-income individuals. Metformin was the most commonly prescribed first-line agent (50.83%) for newly diagnosed T2DM. Approximately 46% of experts recognized dipeptidyl peptidase-4 inhibitors for their superior glycemic durability, with vildagliptin being the preferred agent among 79% of respondents. Vildagliptin was favored by 82% of clinicians for its weight-neutral effects, beta-cell preservation, and favorable side-effect profile. Nearly 76% preferred the 100 mg sustained release (SR) formulation once daily over 50 mg twice daily, citing improved patient compliance. Around 84% of respondents supported the combination of vildagliptin 100 mg SR with metformin SR for enhancing adherence.
Conclusion: The study highlights clinicians’ preference for vildagliptin in the management of T2DM, particularly among middle aged, middle-income patients in Indian settings. Once-daily dosing, favorable efficacy, and improved adherence position vildagliptin, especially in combination with metformin, as a preferred option in routine clinical practice.
Methods: This cross-sectional study was conducted among clinicians across India. The 23-item questionnaire explored various aspects of T2DM management, including demographic patterns, first-line therapy preferences, treatment adherence, perceptions of glycemic durability, and clinical experiences with vildagliptin and its sustained-release formulations. Additional questions addressed socioeconomic trends in diabetes prevalence, factors contributing to nonadherence, and the role of continuous glucose monitoring. Data were analyzed using descriptive statistics.
Results: A total of 242 clinicians participated in the study. The majority (82.64%) identified the highest prevalence of diabetes in the 40–60-year age group, and 71% reported a higher occurrence among middle-income individuals. Metformin was the most commonly prescribed first-line agent (50.83%) for newly diagnosed T2DM. Approximately 46% of experts recognized dipeptidyl peptidase-4 inhibitors for their superior glycemic durability, with vildagliptin being the preferred agent among 79% of respondents. Vildagliptin was favored by 82% of clinicians for its weight-neutral effects, beta-cell preservation, and favorable side-effect profile. Nearly 76% preferred the 100 mg sustained release (SR) formulation once daily over 50 mg twice daily, citing improved patient compliance. Around 84% of respondents supported the combination of vildagliptin 100 mg SR with metformin SR for enhancing adherence.
Conclusion: The study highlights clinicians’ preference for vildagliptin in the management of T2DM, particularly among middle aged, middle-income patients in Indian settings. Once-daily dosing, favorable efficacy, and improved adherence position vildagliptin, especially in combination with metformin, as a preferred option in routine clinical practice.
Introduction
Diabetes remains one of the most widespread chronic diseases
globally. The prevalence of type 2 diabetes mellitus (T2DM) is
increasing rapidly due to factors such as urbanization, population
aging, internal migration, and evolving lifestyles. As of 2019,
approximately 463 million individuals were affected by T2DM
worldwide, with this number projected to rise to 700 million by 2045.
Asia accounts for nearly 60% of global T2DM cases, with China and
India bearing the largest burden [1]. In India alone, prevalence varies
between 2% and 25%, with 77 million cases reported in 2019, a figure
expected to escalate to 134 million by 2045 [2,3]. Key risk factors
include age, ethnicity, obesity, sedentary behavior, and unhealthy
dietary patterns[3]. South Asians, particularly Indian populations, are
more vulnerable, often developing T2DM at younger ages and lower
body mass indices [1]. Major challenges in India include limited
public awareness, inadequate healthcare access, and affordability of
treatment [3].
Pharmacotherapy remains the cornerstone of T2DM
management, with an emphasis on achieving optimal glycemic
control while minimizing adverse effects. Vildagliptin, a dipeptidyl
peptidase-4 inhibitor (DPP-4i), lowers blood glucose levels by
inhibiting the degradation of glucagon-like peptide-1 (GLP-1),
thereby enhancing its activity in both the fed and fasting states. This
results in glucose-dependent stimulation of insulin secretion and
suppression of glucagon release, with a low risk of hypoglycemia or
weight gain. Its prolonged effect is attributed to covalent binding
at the catalytic site of the DPP-4 enzyme [4]. Metformin, the firstline
therapy for T2DM, primarily acts by reducing hepatic glucose
production and improving peripheral insulin sensitivity through
activation of adenosine monophosphate-activated protein kinase
(AMPK). Additionally, it increases endogenous GLP-1 levels,
influences gut glucose metabolism, and modulates the gut microbiota
[5].
The combination of metformin and vildagliptin offers
complementary mechanisms that enhance glycemic control without
increasing the risk of hypoglycemia or weight gain [6].Metformin
enhances vildagliptin’s GLP–1–mediated effects, and the combination,
particularly vildagliptin 50 mg twice daily with metformin, has been
shown to deliver sustained clinical benefits due to vildagliptin's long lasting
enzyme inhibition [7].
This study aims to investigate clinicians' perspectives in the
initiation and management of pharmacotherapy for T2DM, with
a particular focus on the role of vildagliptin and its fixed-dose
combination (FDC) with metformin.
Materials and Methods
We carried out a cross-sectional study among clinicians actively
engaged in routine diabetes management across India from June 2024
to December 2024. The study was conducted after receiving approval
from Bangalore Ethics, an Independent Ethics Committee, which was
recognized by the Indian Regulatory Authority, the Drug Controller
General of India.
An invitation was sent to leading diabetologists in managing
T2DM patients in the month of March 2024 for participation in
this Indian survey. About 242 clinicians from major cities of all
Indian states, representing the geographical distribution, shared
their willingness to participate and provide necessary data. The
questionnaire booklet titled VERGE (Evaluate the Vildagliptin
Extended-Release Dosage and to Gather Insights from the Experts)
study was sent to clinicians who were interested to participate. The
VERGE study questionnaire consisted of 23 questions designed to
gather clinical perspectives and experiences regarding various aspects
of diabetes care. It specifically focused on addressing demographic
patterns of diabetes, first-line therapy preferences, pharmacotherapy
adherence, perceptions of glycemic durability, and clinical
experiences with vildagliptin and its sustained-release formulations.
Additional questions explored socio-economic trends in diabetes
prevalence, factors contributing to treatment non-adherence, and the
role of continuous glucose monitoring, with a particular emphasis on
the use of vildagliptin and its FDC with metformin. Clinicians had the
option to skip any questions they preferred not to answer. They were
instructed to complete the questionnaire independently, without
consulting their colleagues. Written informed consent was obtained
from all participants before the study commenced.
Statistical Analysis:
Descriptive statistical methods were used to analyse the data.
Categorical variables were summarized as frequencies and percentages
to represent distribution patterns. Visual representations, including
pie and bar charts, were created using Microsoft Excel 2013 (version
16.0.13901.20400) to support the interpretation of the findings.Results
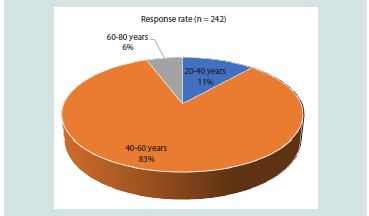
The study included 242 participants, with the majority (82.64%)
indicating that diabetes is more prevalent in the 40–60-year age group
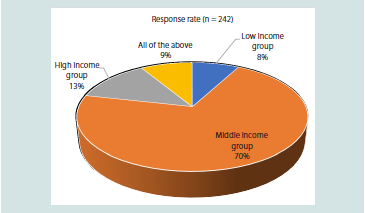
[Figure 1]. A significant proportion (70.66%) of clinicians reported
that the disease is more commonly seen among individuals belonging
to the middle-income economic group in their practice [Figure 2].
Over half (51.24%) of the participants estimated that 30- 40% of individuals with T2DM are likely to be non-adherent to pharmacotherapy. Approximately 68% of the experts highlighted that multiple dosing, polypharmacy, and adverse events are common causes of non-adherence to pharmacotherapy. About 37% of participants identified poor medication adherence as a key challenge when initiating pharmacotherapy in T2DM.
Around 35% of the respondents reported that they use continuous glucose monitoring (CGM) as a tool for 10% of patients when starting pharmacotherapy. About 62% of participants stated they consider glycemic status and associated complications when initiating pharmacotherapy in T2DM. More than half (50.83%) of the
Over half (51.24%) of the participants estimated that 30- 40% of individuals with T2DM are likely to be non-adherent to pharmacotherapy. Approximately 68% of the experts highlighted that multiple dosing, polypharmacy, and adverse events are common causes of non-adherence to pharmacotherapy. About 37% of participants identified poor medication adherence as a key challenge when initiating pharmacotherapy in T2DM.
Around 35% of the respondents reported that they use continuous glucose monitoring (CGM) as a tool for 10% of patients when starting pharmacotherapy. About 62% of participants stated they consider glycemic status and associated complications when initiating pharmacotherapy in T2DM. More than half (50.83%) of the
Figure 1: Distribution of responses regarding the most prevalent age group
for diabetes in clinical practice
Figure 2: Distribution of responses on the prevalence of diabetes among
socio-economic status groups in clinical practice
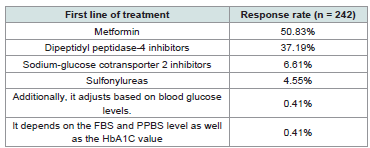
clinicians reported that metformin is the first-line treatment for newly
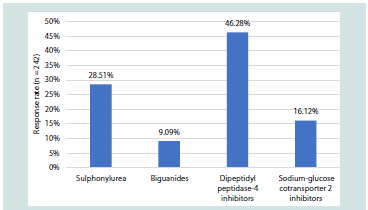
diagnosed diabetes in their practice [Table 1]. Around 46% noted that
DPP-4i provides greater glycemic durability as monotherapy after
initiating oral anti-diabetic drugs (OADs) [Figure 3].
About 45% of experts reported that DPP-4 inhibitors are typically
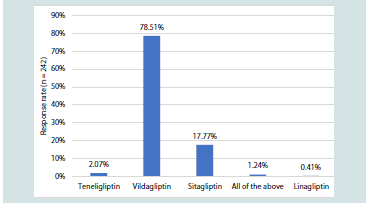
initiated after the failure of monotherapy. The majority (78.51%)
preferred vildagliptin as the DPP-4 inhibitor of choice in clinical
practice [Figure 4]. Approximately 44% of participants indicated that
10–30% of patients in their practice are prescribed vildagliptin. Most
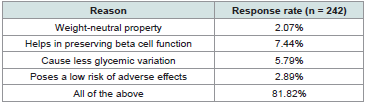
clinicians (81.82%) favored vildagliptin over other agents due to its
weight-neutral properties, ability to preserve beta-cell function, lower
glycemic variability, and minimal adverse effects [Table 2] .
Nearly half of the participants (49.59%) reported observing
a 1–1.5% reduction in HbA1c levels following the initiation of
vildagliptin in clinical practice. About 53% of respondents indicated
that they prescribe vildagliptin 100 mg SR in most patients.
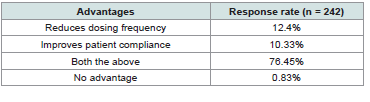
Approximately 76% of experts highlighted the advantages of
vildagliptin 100 mg SR once daily over vildagliptin 50 mg twice daily,
citing reduced dosing frequency and improved patient compliance
[Table 3].
Approximately 43% of respondents reported that 11–25% of patients in their practice are started on the vildagliptin 100 mg SR + metformin SR formulation as an initiation strategy. Around 52%
Approximately 43% of respondents reported that 11–25% of patients in their practice are started on the vildagliptin 100 mg SR + metformin SR formulation as an initiation strategy. Around 52%
Table 1: Distribution of responses on first-line treatment for newly diagnosed
diabetes in clinical practice
Figure 3:Distribution of responses on clinicians’ perceptions of glycemic
durability of OAD classes as monotherapy
Table 3: Distribution of responses on perceived advantages of vildagliptin 100
mg SR once daily compared to 50 mg twice daily
Table 4:Distribution of responses on reasons supporting the use of vildagliptin
100 mg SR + metformin SR as an initiation strategy
of clinicians identified patients aged between 40 and 50 years as
the preferred age group for initiating vildagliptin 100 mg SR and
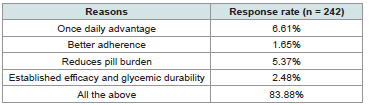
metformin combination therapy. The majority (83.88%) cited the
benefits of once-daily dosing, improved adherence, reduced pill
burden, and established glycemic efficacy as key reasons for selecting
vildagliptin 100 mg SR + metformin SR as an initiation strategy
[Table 4] .
In day-to-day clinical practice, around 70% of participants
preferred using vildagliptin 100 mg SR + metformin 500 mg SR and
vildagliptin 100 mg SR + metformin 1000 mg SR combinations.
About 58% of participants reported better glycemic efficacy with
the FDC of vildagliptin and metformin in young, elderly, and longstanding
diabetic patients.
Approximately 69% of participants observed a difference
in glycemic efficacy between the twice-daily vildagliptin 50 mg
immediate-release formulation and the once-daily vildagliptin 100
mg SR formulation in only a few patients. Around 63% reported a
1–1.5% reduction in HbA1c with the vildagliptin SR + metformin SR
combination.
Discussion
The study provides valuable insights into the clinician's
preferences and prescribing patterns of clinicians managing T2DM
in Indian settings. The majority of participants reported that diabetes
is most prevalent in the 40–60-year age group. Supporting this,
Naveed et al. observed a significant increase in diabetes risk among
individuals aged 31–60 years [8].Similarly, Awan et al. reported a 38%
prevalence of diabetes in individuals aged 40 and above, with higher
rates among males and those in the 50–60 age group [9].Cheng et al.
reported a 75% increase in diabetes cases from 1988-1994 to 2005-
2010, with middle-aged adults contributing 52.9% to this rise [10].
Mayega et al. highlighted a high prevalence of diabetes among those
aged 35-60 years [11].
A significant proportion of clinicians studied reported that
diabetes is more commonly observed among individuals from the
middle-income socioeconomic group in their practice. Supporting
this observation, Deepa et al. documented a rapid reversal of the
socioeconomic gradient for diabetes risk factors in urban India, with
prevalence rates converging between middle- and low-income groups
[12]. Hydrie et al. highlighted that, although children in both groups
exhibited, middle-income children had a significantly increased risk
for diabetes [13]. Further highlighting the evolving landscape, Mailti
et al. analyzed data from the NFHS-5 (2019–2021) and revealed that
the age- and sex-adjusted prevalence of diabetes among adults aged
15 years and older rose from 13.1% in the poorest wealth quintile to
18.8% in the richest quintile [14].
Over half of the study participants reported that metformin is the
first-line treatment for newly diagnosed diabetes in their practice. This
aligns with longstanding clinical guidelines from major organizations
such as the American Diabetes Association (ADA) and the European
Association for the Study of Diabetes (EASD), which have consistently
recommended metformin as the initial pharmacologic therapy for
T2DM due to its proven efficacy, safety profile, and cost-effectiveness
[15,16]. According to Baker et al. and Ahmad et al., metformin
remains the most commonly prescribed glucose-lowering therapy
(GLT) worldwide and continues to be recommended as first-line
treatment for newly diagnosed T2DM. This is supported by clinical
guidelines and evidence from the UK Perspective Diabetes Study
(UKPDS), which demonstrated cardiovascular mortality benefits in
overweight individuals treated with metformin [17,18].
Many study participants noted that DPP-4i offer greater glycemic
durability as monotherapy after initiating OADs. A meta-analysis of
long-term randomized controlled trials demonstrated that DPP-4
inhibitors are associated with significantly better glycemic durability
compared to sulfonylureas, as evidenced by smaller increases in
HbA1c levels over a 104-week treatment period. This suggests
that DPP-4 inhibitors may better preserve islet β-cell function,
contributing to sustained glycemic control [19]. Esposito et al. also
noted that DPP-4i are effective in reducing HbA1c in the first year of
treatment [20].
The majority of respondents preferred vildagliptin as their DPP-4i
of choice in clinical practice. This aligns with findings from Matheiu et
al., who noted that vildagliptin is among the most extensively studied
DPP-4is, demonstrating strong clinical utility and safety in managing
T2DM [21].Saini et al. also highlighted that vildagliptin significantly
increases post-meal active plasma GLP-1 levels by 1.5 to 3 times
compared to placebo. A 100-mg dose of vildagliptin is sufficient to
fully suppress DPP-4 activity in patients with T2DM [22].
Clinicians favored vildagliptin over other agents primarily
due to its weight-neutral properties, ability to preserve beta-cell
function, lower glycemic variability, and minimal adverse effects.
Foley and Jordan noted that while DPP-4i are generally weight neutral,
vildagliptin has been associated with modest weight loss in
patients with relatively low baseline glycemia [23]. Foley et al. found
that one-year treatment with vildagliptin significantly improved
beta-cell secretory capacity, though this effect was not sustained
after discontinuation [24]. Panina highlighted that vildagliptin is
a potent, selective DPP-4i that enhances islet alpha- and beta-cell
responsiveness to glucose [25]. Pan and Wang added that vildagliptin
is well-tolerated with a low incidence of adverse events and does not
increase the risk of cardiovascular or cerebrovascular events [26].
Many participants highlighted the benefits of vildagliptin 100 mg
SR once daily over vildagliptin 50 mg twice daily, such as reduced
dosing frequency and enhanced patient compliance. Sangana et al.
confirmed the therapeutic equivalence of the IR and SR formulations
in terms of DPP-4 enzyme inhibition, suggesting that the 100 mg SR
formulation may enhance treatment compliance [27]. Warrier et al.
demonstrated that once-daily vildagliptin SR 100 mg is bioequivalent
to twice-daily vildagliptin IR 50 mg. The 100 mg SR formulation
provides over 80% DPP-4 inhibition for 24 hours, which may lead to
a meaningful glucose-lowering effect while reducing the pill burden
for patients with diabetes [28].
The majority of clinicians cited the benefits of once-daily dosing,
better adherence, reduced pill burden, and proven glycemic efficacy as
key reasons for selecting vildagliptin 100 mg SR + metformin SR as an
initiation strategy. Chatterjee and Chatterjee found that a once-daily
metformin-vildagliptin combination significantly reduced plasma
glucose and HbA1c, making it a viable, cost-effective alternative to
a twice-daily regimen [29].A review with real-world case reports by
Chawla et al. emphasized that early initiation of combination therapy
helps achieve glycemic goals faster, with metformin SR–vildagliptin
FDC offering better tolerability, fewer adverse events, and improved
compliance compared to the metformin IR–vildagliptin FDC[30].
This study offers valuable clinical insights into current clinical
practices in the management of T2DM, with responses from 242
clinicians highlighting trends in disease prevalence, treatment
preferences, and adherence challenges. It effectively captures
practical perspectives on the use of metformin and DPP-4 inhibitors,
particularly vildagliptin, and underscores key factors influencing
clinicians’ therapeutic choices, such as efficacy, safety, dosing
convenience, and patient compliance. These findings align with
existing clinical guidelines, adding relevance and applicability to dayto-
day practice. However, the study’s reliance on self-reported data
introduces the possibility of recall and selection bias, and the lack of
information on geographic distribution, methodology, and objective
patient outcomes limits the generalizability of the survey findings.
Conclusion
The study highlights clinicians’ perspectives in managing T2DM,
with metformin remaining the cornerstone of therapy and vildagliptin
favored for its efficacy, safety, and convenience. The preference for
once-daily dosing and fixed-dose combinations supports better
adherence and glycemic control. Overall, early combination therapy
appears to be a practical and effective strategy in routine diabetes care.
Acknowledgement
We would like to thank all the clinicians who were actively
participating in this study.
Author contributions
Both authors have contributed equally to the development of the manuscript.
Disclosure of compliance with ethical principles
The study was conducted after receiving approval from Bangalore Ethics, an Independent Ethics Committee, which was recognized by the Indian Regulatory Authority, Drug Controller General of India.
Author contributions
Both authors have contributed equally to the development of the manuscript.
Disclosure of compliance with ethical principles
The study was conducted after receiving approval from Bangalore Ethics, an Independent Ethics Committee, which was recognized by the Indian Regulatory Authority, Drug Controller General of India.