Advances in Diabetes & Endocrinology
Download PDF
Research Article
*Address for Correspondence: Dror Robinson, Department of Orthopedics, Rabin Medical Center, Hasharon Hospital, Petah Tikwa, affiliated with Tel Aviv University, Israel, E-mail: dror61@gmail.com
Citation: Robinson D, Yassin M. Effect of Addition of Medicinal Cannabis Treatment to Conventional Treatment in Diabetic Neuropathy Patients. Adv Diabetes Endocrinol 2016; 1(1): 5.
Copyright © 2016 Robinson D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Advances in Diabetes & Endocrinology | Volume: 1, Issue: 1
Submission: 11 October, 2016 | Accepted: 19 November, 2016| Published: 26 November, 2016
Presence of diabetic polyneuropathy per EMG diagnosis and neurologist consultation. Pain severity (BPI) of at least 7 or higher at both screening and the MCT initiation visit (3 months). Ineffective treatment for at least one year by conventional medication including opiates and at least one of the atypical analgesics.
Exclusion criteria:Psychiatric condition making MCT treatment too risky in the judgment of a psychiatrist. Severe heart failure (NYHA grade 3 or above). Severe respiratory insufficiency [GOLD staging uses four categories of severity for COPD (http://www.webmd.com/lung/copd/gold-criteria-for-copd), stage 3 or higher were excluded].
Effect of Addition of Medicinal Cannabis Treatment to Conventional Treatment in Diabetic Neuropathy Patients
Mustafa Yassin and Dror Robinson*
- Department of Orthopedics, Hasharon Hospital and Tel Aviv University, Israel
*Address for Correspondence: Dror Robinson, Department of Orthopedics, Rabin Medical Center, Hasharon Hospital, Petah Tikwa, affiliated with Tel Aviv University, Israel, E-mail: dror61@gmail.com
Citation: Robinson D, Yassin M. Effect of Addition of Medicinal Cannabis Treatment to Conventional Treatment in Diabetic Neuropathy Patients. Adv Diabetes Endocrinol 2016; 1(1): 5.
Copyright © 2016 Robinson D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Advances in Diabetes & Endocrinology | Volume: 1, Issue: 1
Submission: 11 October, 2016 | Accepted: 19 November, 2016| Published: 26 November, 2016
Abstract
Background: Diabetic neuropathy is unfortunately the most common complication associated with a common malady increasing in prevalence by 5% a year. None of the disease-modifying drugs that have been designed to target multiple metabolic pathways has proven effective. Thus the treatment currently is mostly symptomatic. The current study assessed the ability of cannabis added to maximal conventional therapy to ameliorate diabetic neuropathy symptoms.Methods: At screening, patients were assessed for possibility of being treated by receiving cannabis therapy (CT, smoking, 20 grams per month) provided that they have completed a period of at least 12 months of optimal conventional treatment including at least one narcotic agent and at least one of following analgesic treatments: tricyclic antidepressants (amitriptyline and nortriptyline), anticonvulsants (gabapentin and pregabalin), and Serotonin- Norepinephrine Reuptake Inhibitors (SNRIs) (duloxetine and venlafaxine). Patients suitable for CT, were treated for at least 3 months more by alpha lipoic acid 600 mg, vitamin B complex, duloxetine 30 mg and tramadol 100 mg up to thrice a day. If after 3 months of treatment, the patients were still painful (BPI pain severity higher than 7) cannabis therapy was begun. Patients were followed up every 6 months for up to one year. The following Patient Reported Outcome scores (PRO’s) were collected: VAS pain intensity, VAS pain severity, BPI pain severity, BPI pain interference and SF12.
Results: 89 patients were screened. 15 of them improved after 3 months of maximal conventional therapy, and thus did not meet the inclusion criteria of minimal pain severity. 74 patients begun cannabis therapy and results are available after 6 months for 73 of them and 70 patients were followed up to 12 months. 4/74 patients stopped CT (1/74 due to ileus, 3/74 due to pain resolution). BPI pain severity decreased from 9.4±0.8 to 4.3±1.7 at six months, while pain interference decreased from 8.6±1.1 to 3.7±1.6. SF12v2 Physical Compounded Score (PCS) changed from 34±8 to 46±8 at 6M.
Conclusions: The addition of CT leads to substantial pain reduction accompanied by decrease in HbA1C while quality of life questionnaires scores improved. The mechanism leading to improved diabetic control is not known at this time and requires further study.
Keywords
Cannabis; Diabetic neuropathy; Diabetes Mellitus; Glycosylated hemoglobin; OpiatesIntroduction
Diabetes mellitus is increasing in prevalence in the western world. Its estimated prevalence currently is about 30 percent of diabetic people making it the most common complication of diabetes [1]. Currently few treatments are available, and they are mostly composed of pain relieving medications [2]. The guidelines recommend using opiates, local lidocaine as well as duloxetine, pregabalin, gabapentin, and tricyclic antidepressants as the mainstays of treatment [3]. As none of these medications is curative, there is a place for exploration of other medications [4]. One theory is that metabolic correction might improve diabetic neuropathy and not only alleviate symptoms [5]. Metabolic correction requires the administration of several vitamins and metabolites including lipoic acid, acetyl-Lcarnitine, benfotiamine and the combination of active B vitamins L-methylfolate, methylcobalamin and piridoxal-6-phosphate. Insulin therapy has been associated with increased rates of polyneuropathy and a syndrome of treatment induced polyneuropathy has been described. It has been suggested that the elevated levels of insulin, while lowering glucose levels actually worsen the metabolic state of the individual, in fact amelioration of metabolic balance has been shown to improve neuropathy [6]. Of some interest are the findings that cannabis directly improves glucose metabolism by lowering insulin levels [7], as well as BMI. This observation led us to explore the possibility of improving diabetic polyneuropathy symptoms using Medicinal Cannabis Therapy (MCT).Methods
Diagnosis of neuropathyAll patients were referred by either their family physician or a pain specialist/neurologist for consideration of CT. All had electrophysiologic tests to confirm Diabetic Polyneuropathy (DPN).
Neurophysiologic tests: Nerve conduction studies were taken as a gold standard for the diagnosis of DPN in this study. All the tests were recorded by a 4-channel EMG device, including sensory NCV of median nerve in both upper extremities and superficial peroneal nerve in both lower extremities, motor NCV of common peroneal nerve, M-wave of median nerve, F-waves and H-reflexes of tibial nerve. The skin temperature at the legs was maintained at or above 30 °C. Based on NCV and EMG findings compared with the normal values in the specific EMG institute, DPN was confirmed or excluded in each patient by a specialist neurologist.
Objective scoring of neuropathy: The DNE score was used to score the severity of neuropathy [8]. The score has a maximum 16 point score indicating severe dysfunction. Scoring was performed at screening, MCT initiation and at the 6 months follow-up. In addition vibration threshold was assessed in all patients in a quantitative manner over the right big toe using a Biothesiometer (http://www.biothesiometer.com/) using the 5 point step elevation method beginning at 0. Each patient was tested 3 times and results were averaged.
Study design
The national regulations of usage of medicinal cannabis demand that a patient be treated by a specialist (pain specialist, neurologist or orthopedic surgeon) for a period of at least one year, prior to implementation of MCT. A recent survey showed that DPN was treated with anticonvulsants in 27%, SNRIs in 18%, and opioids in 43% [9]. In order to make sure that all patients were treated by MCT after optimal therapy of conventional medication, the study design was an open label, single center and cross-over study. The patients considered for inclusion in the trial were screened for inclusion, and treated for at least three months period by a standardized treatment regimen including tramadol 100 mg once to thrice a day as well as duloxetine 30 mg once a day. In addition alpha-lipoic acid 600 mg per day, vitamins B1, B6 and B12 were administered during the same period. Patients in whom pain severity score was not less than 7 after this optimal therapy period (all have previously received at least one year of non-standardized pain medication) were treated by MCT therapy. MCT therapy was standardized with a dosage of smoking 20 gram per month. High THC strain was recommended for evening usage and high CBD strain for use during the day (if needed). Previous medical therapy was allowed according to patient choice. The usage of concomitant medications was assessed using pharmacy issued reports.
Follow-up was every 4-6 months up to one year. The following patient reported outcome scores (PRO’s) were collected at each visit: VAS pain intensity, VAS pain frequency, SF12v2, BPI pain severityand pain interference. In addition fasting glucose levels and HbA1C were collected at screening and after six months and 12 months.
Comorbidities: These patients are mostly likely to have multiple comorbidities. All patients were interviewed by one of the authors and their family physician issued a detailed medical history summary. These two modalities allowed to assess known comorbidities of these patients. Due to the risk involved in MCT therapy in any case of cardiac disease, a cardiologist assessed the patient risk of being damaged by MCT, and ruled out severe congestive heart failure. Patients with respiratory disease (particularly COPD) were assessed by a pulmonologist to assess the risk of MCT therapy. Any patient with previous psychiatric disorder or history of drug abuse was assessed by a psychiatrist specializing in drug-abuse, to assess the risk of MCT in this specific patient. Only patients who were approved by the relevant specialists were MCT treated.
Dosage increase: All patients were started on a fixed 20 gram monthly regimen. Dose increase was considered only after at least 4 months of treatment provided a self-reported usage table indicated shortage at the end of the month. Dosage increase was to 30 grams per month. Further dosage increase was considered after at least 9 months of MCT.
Concomitant medications: Concomitant pharmaceutical usage was assessed according to pharmacy reports of issued medications. Results are reported in morphine equivalent units according to a published scale [10].
Adverse events: Adverse events were collected at each visit according to a fixed list of known MCT related side effects including (according to Ministry of Health requirements): eye redness, dizziness, anxiety, weight gain, euphoria, lethargy, increased intraocular pressure, confusion, dispersonalization, uncontrolled laughter, drowsiness, psychosis, abnormal color perception, abnormal noise sensitivity, feeling of superiority, hunger, loss of temporal perception, hallucinations, intrusive thoughts, palpitations, decreased eye sight, depression, dry mouth, other side effect.
Inclusion criteria:
Endpoints
Primary endpoint:Change in Average BPI score – first 4 items
Change in VAS score (intensity and frequency) Change in SF12v2 PCS Change in SF12v2 MCS Change in % HbA1C Change in fasting glucose levels
Statistical analysis
Secondary endpoint:
Statistical analysis was performed by one of the authors (D.R.) using the analyse-it Excel add-in (version 2.30, Excel 12+, 2016, Analyse-it Software Ltd). Demographics parameters were described as mean±standard deviation; t-test was used for gender comparisons and for comparison of baseline measures to last available follow-up (LAF) measures. The significance level was 0.05. Differences that did not reach that level were described as n.s. ANOVA for repeated measures was used for repetitious scores at various time points.
Results
Study cohort89 patients were considered for inclusion. 15 patients were not treated by MCT (5 improved to below the pain threshold, 4 excluded due to prior psychiatric disease, 3 patients excluded due to cardiovascular or pulmonary problems, 3 due to insurance coverage issues). 74 patients were started on MCT therapy all of whom completed the 6 months treatment. 4 patients dropped out between the 6 months and 12 months visits (1 due to improvement, 1 due to inability to smoke cannabis, 2 were lost to follow-up). 70 patients were available for analysis at the 12 months visit.
Demographics
MCT was begun in 74 patients (23 females and 51 males). The average age was 63.8±13 (range 27-88) years with females significantly older 70.8±4.8 vs. men 60.8±14.6. Comorbidities are delineated in Table 1.
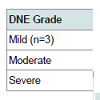
Neuropathy evaluation: Baseline Biothesiometer reading ranged from 4 to 50 (normal range at the great toe is up to 6) and averaged 9.2±1.8 in females and 8.8±2 in males (n.s.). Baseline DNE score ranged from 4-12 (8.9±1.8). Patients cohort was split arbitrarily into three groups (severe neuropathy DNE score over 8 (n=58), mild neuropathy with DNE score less than 6 (n=3) and a moderate group in between (n=13) (Table 2).
The correlation between DNE score and Biothesiometer results was 0.38 [Pearson r coefficient, 95% Confidence Interval (CI) 0.16-0.56, p<0.001].
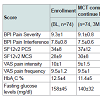
Baseline PRO’s
Table 3 delineates the baseline characteristics of the enrolled patients and the MCT treated group. PRO’s scores were similar for the treated group and the drop-off group except for a slightly (n.s.) lower pain scores for the latter group at MCT commencement time point (3M). Quality of life scores were similar in drop-outs and those who went on to receive the MCT. Scores improved slightly from enrollment to MCT commencement 3 months later (n.s.).
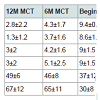
Change over time in PRO’s: MCT led to significant decline in pain severity score and pain interference score over time. Most of the decrease occurred over the first six months, but pain decline continued over the next six months to the one year follow-up visit (Table 4). The VAS pain intensity and pain frequency followed a similar pattern. The SF12v2 PCS and MCS improved as well. The change in the following PRO’s (BPI pain severity, BPI pain interference, VAS intensity, VAS frequency, SF12v2 PCS and SF12v2 MCS) overtime was found highly significant (one-way ANOVA, p<0.001) Using pairwise against control (MCT beginning time point) one-way ANOVA, the difference between enrollment and beginning of MCT therapy 3 months later was not significant, but the difference between beginning MCT therapy and 6M and 12M time points was significant (Dunnett contrast pairwise comparison).
Change in concomitant medication: As per study design all patients had begun MCT treatment while receiving for at least 3 months tramadol and duloxetine. Some patients received other opiates. Opiate treatment at baseline averaged 37.8±29.3 and declined to 4.2±14.2 at 12M. Duloxetine use declined from 74/74 patients at the beginning to 7/74 at 12M (notice that this analysis includes the 4 patients who declined to continue MCT therapy as they continued to be followed-up at the office.
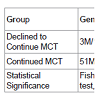
Comparison of MCT-decline group to MCT-continue group: Patients were evaluated at each visit by PRO’s. 4 patients declined to continue MCT after 6 months. The reason for stopping treatment was physician recommendation in one case that developed ileus. The patient has been treated due to autonomic and peripheral diabetic neuropathy, and had a history of five previous operations due to intestinal obstruction secondary to Crohn’s disease. The relation of the MCT to the ileus remains unclear, but for safety reasons it was decided not to continue the MCT. 3 other patients stopped the MCT due to pain resolution (Table 5). These 4 patients had similar age distribution and pain severity score to the other group, but had more pain interference at baseline and more decline in pain severity at 6 months relative to the group who continued MCT. Due to the small MCT-decline group size it is possible that other differences were not revealed in this cohort.
MCT safety
A single serious adverse event that led to hospitalization was encountered as described above (ileus). Its relation to the MCT therapy remains unclear as the patient had multiple previous incidents of intestinal obstruction.
Adverse events encountered included 18 incidents of red eyes, 45 incidents of increased appetite, on the other hand 70/74 patients reported better sleep at night, 23/74 patients reported less drowsiness and 62/74 patients reported better ability to concentrate. No psychiatric symptoms induced by MCT were encountered though 57/74 patients reported reduced anxiety.
Changes in glycemic control were an unexpected result of the MCT therapy. Fasting glucose levels declined from 186±38 mg/dl at MCT beginning to 160±25 mg/dl at 12M (n.s.). However a surprising finding was a change in HbA1C from 12.8±4% to 8.4±3% at 12M (n=74, t-test, p<0.01).
Discussion
The current study appears to indicate that addition of CT therapy in diabetic neuropathy patients leads to reduction of pain and improvement of quality of life PRO’s. Concomitantly there is a trend to improvement of fasting glucose levels and better longterm glucose balance as evidenced by HbA1C levels. Further study is needed in order to explore whether MCT therapy leads to decrease in insulin levels, which might also explain the reduction in neuropathic symptoms. Previously it was reported that current marijuana use was associated with 16% lower fasting insulin levels [11]. The better glucose metabolism in the polyneuropathy group contrasts with previous suggestions based on preclinical studies, that cannabis treatment increases fat deposition due to CB1 receptor activation [12].It seems that while in animals, cannabis (delivered as food additive) leads to hyperphagy and obesity, there is in humans an inverse cannabis smoking-diabetes mellitus association [13]. Thisindicates that smoking might be the correct method of delivering cannabis in humans despite the obvious adverse implications of recommending patients to smoke.
The opiate sparing effect of cannabis has been previously described [14]. While the atypical analgesics were initially considered to have a low abuse potential, recently abuse of pregabalin has been described [15]. Thus, the duloxetine sparing effect appears also to be significant from an abuse perspective. In addition duloxetine is a rather expensive pharmaceutical agent costing approximately $150 per month [9]. The replacement of opiates and duloxetine by MCT appears to be also cost-effective therapy.
Further clinical research is necessary in order to define whether long term MCT will lead to improved nerve function, as the time frame of this study is too short to allow nerve regeneration.
References
- Aslam A, Singh J, Rajbhandari S (2014) Pathogenesis of painful diabetic neuropathy. Pain Res Treat 2014: 1-7.
- Javed S, Alam U, Malik RA (2015) Treating diabetic neuropathy: present strategies and emerging solutions. Rev Diabet Stud 12: 63-83.
- Ziegler D, Fonseca V (2015) From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complications 29: 146-156.
- Shenoy AM (2012) Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn) 18: 192-198.
- Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, Allende-Vigo MZ, Duconge J (2011) Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol 6: 260-273.
- Fedele D, Bellavere F, Cardone C, Ferri M, Crepaldi G (1985) Improvement of cardiovascular autonomic reflexes after amelioration of metabolic control in insulin-dependent diabetic subjects with severe autonomic neuropathy. Horm Metab Res 17: 410-413.
- Ngueta G, Belanger RE, Laouan-Sidi EA, Lucas M (2015) Cannabis use in relation to obesity and insulin resistance in the Inuit population. Obesity (Silver Spring) 23: 290-295.
- Meijer JW, van Sonderen E, Blaauwwiekel EE, Smit AJ, Groothoff JW, et al. (2000) Diabetic neuropathy examination: a hierarchical scoring system to diagnose distal polyneuropathy in diabetes. Diabetes Care 23: 750-753.
- Juster-Switlyk K, Smith AG (2016) Updates in diabetic peripheral neuropathy. Version 1 5: F1000Res.
- Kishner S, Windle ML, Schraga ED (2016) Opioid equivalents and conversions.
- Penner EA, Buettner H, Mittleman MA (2013) The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 126: 583-589.
- Kirkham T (2008) Endocannabinoids and the neurochemistry of gluttony. J Neuroendocrinol 20: 1099-1100.
- Alshaarawy O, Anthony JC (2015) Cannabis smoking and diabetes mellitus: results from meta-analysis with eight independent replication samples. Epidemiology 26: 597-600.
- Lucas P (2012) Cannabis as an adjunct to or substitute for opiates in the treatment of chronic pain. J Psychoactive Drugs 44: 125-133.
- McNamara S, Stokes S, Kilduff R, Shine A (2015) Pregabalin abuse amongst opioid substitution treatment patients. Ir Med J 108: 309-310.