Journal of Pediatrics & Child Care
Download PDF
Review article
*Address for Correspondence: Ashraf M Aly, MD, PhD, Department of Pediatric Cardiology, University of Texas Medical Branch, Galveston, TX-77550, USA, Tel: 409-772-2341; E-mail: amaly@utmb.edu
Citation: Dasgupta S, Jain SK, Aly AM. Neonatal Hypotension, the Role of Hydrocortisone and Other Pharmacological Agents in its Management. J Pediatr Child Care. 2016;2(1): 08.
Copyright © 2016 Aly, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pediatrics & Child Care | ISSN: 2380-0534 | Volume: 2, Issue: 1
Submission: 01 December, 2015 | Accepted: 25 February, 2016 | Published: 03 March, 2016
Reviewed & Approved by: Dr. Krishna Dummula, Division of Neonatology, University of Kansas School of Medicine, USA
Although hydrocortisone is usually effective orally, other routes of administration can be advantageous. Certain water-soluble esters of hydrocortisone maybe administered intravenously to attain rapid and high concentrations in the body fluids. In addition, intramuscular administration leads to a prolonged duration of action of hydrocortisone. After absorption, 90% of hydrocortisone is bound to proteins (corticosteroid binding globulin (CBG) and albumin) and only the 10% unbound fraction is free to enter the cells to mediate its effects. At low concentrations, most of the hydrocortisone is protein bound, leading to reduced effects. In contrast, when the binding capacity of the proteins is exceeded at higher concentrations of hydrocortisone, free-state hydrocortisone is available to mediate physiologic action. Hydrocortisone is metabolized mostly in the liver and to a lesser degree in the kidney yielding water-soluble sulfate esters and glucouronides, which are then mainly excreted in the urine [30].
Neonatal Hypotension, the Role of Hydrocortisone and Other Pharmacological Agents in its Management
Soham Dasgupta1, Sunil K Jain2 and Ashraf M Aly3*
- 1Department of Pediatrics, University of Texas Medical Branch, Galveston, TX-77550, USA
- 2Department of Neonatology, University of Texas Medical Branch, Galveston, TX-77550, USA
- 3Department of Pediatric Cardiology, University of Texas Medical Branch, Galveston, TX-77550, USA
*Address for Correspondence: Ashraf M Aly, MD, PhD, Department of Pediatric Cardiology, University of Texas Medical Branch, Galveston, TX-77550, USA, Tel: 409-772-2341; E-mail: amaly@utmb.edu
Citation: Dasgupta S, Jain SK, Aly AM. Neonatal Hypotension, the Role of Hydrocortisone and Other Pharmacological Agents in its Management. J Pediatr Child Care. 2016;2(1): 08.
Copyright © 2016 Aly, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Pediatrics & Child Care | ISSN: 2380-0534 | Volume: 2, Issue: 1
Submission: 01 December, 2015 | Accepted: 25 February, 2016 | Published: 03 March, 2016
Reviewed & Approved by: Dr. Krishna Dummula, Division of Neonatology, University of Kansas School of Medicine, USA
Abstract
Neonatal hypotension remains one of the most commonly encountered problems in neonatal intensive care units. Pharmacologic agents used in the management of neonatal hypotension include volume expanders, inotropes and vasopressors. Hydrocortisone can be used in resistant cases. Hydrocortisone acts both via genomic (slow) and non-genomic (fast) effects, however it is not recommended for urgent control of hypotension in neonates because its effects are usually delayed. Although the addition of hydrocortisone for the treatment of neonatal hypotension improves clinical outcomes, prolonged use of hydrocortisone may lead to suppression of the hypothalamic-pituitary axis and should be weaned gradually. Hydrocortisone in minimal dosages and for the shortest duration of therapy may prevent potential short and long-term side effects of its use.Abbreviations
PDA: Patent Ductus Arteriosus; HPA: Hypothalamic-Pituitary Axis; NEC: Necrotizing Enterocolitis; TSH: Thyroid Stimulating Hormone; ELBW: Extremely Low Birth Weight; INO: Inhaled Nitric Oxide; PPHN: Persistent Pulmonary Hypertension of The Newborn; ACTH: Adrenocorticotrophic Hormone; CRH: Corticotrophin Releasing Hormone; AVP: Arginine-Vasopressin; CBG: Corticosteroid Binding Globulin; GRE: Glucocorticoid Response Elements; VLBW: Very Low Birth Weight; TAP: Transient Adrenocortical Insufficiency of Prematurity; CIRCI: Critical Illness Related Corticosteroid Insufficiency; HPA: Hypothalamic Pituitary AdrenalDefinition of Hypotension
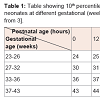
The establishment of normal blood pressure ranges specific to gestational and postnatal age remains an elusive goal [1]. One suggested definition for neonatal hypotension is any value that falls below the tenth percentile for gestational and postnatal age (Table 1) [2,3]. Cayabyab et al. defines hypotension as any systolic blood pressure at which there is loss of auto-regulation of organ blood flow resulting in tissue ischemia [2]. Shead provides yet another working definition by suggesting that a mean blood pressure less than the neonate’s gestational age can be considered hypotension [4]. Hence, there is no clear consensus on the definition for neonatal hypotension. Currently, proper diagnosis and management of neonatal hypotension necessitates the use of clinical judgment following a careful review of physiological parameters.Causes of Neonatal Hypotension
Neonatal hypotension is most commonly seen in premature neonates and its incidence is inversely related to the gestational age at delivery [5]. The causes of hypotension in both term and preterm neonates are summarized in Table 2.Hypotension in preterm infants is predominantly due to either abnormal peripheral vaso-regulation or myocardial dysfunction. Hypovolemia as a primary cause is observed less frequently [6]. Since the autonomic nervous system that regulates the peripheral systemic resistance is immature in preterm infants [7], these patients are likely to develop peripheral vasodilatation and hypotension [6]. In addition, the immature myocardium of a preterm infant also has less mitochondria than a term infant. This decreases the ability of the myocardium to adapt from pumping against a low resistance circuit (placenta) to pumping against a high resistance circuit (systemic circulation) after delivery, thus leading to hypotension [4,8].
Some other etiologies of hypotension in this population include both physiologic/anatomic factors as well as pathologic factors. Physiologic factors include the presence of a patent ductus arteriosus (PDA) and relative adrenal insufficiency secondary to an immature hypothalamic-pituitary axis (HPA) [9]. Pathologic factors include perinatal depression, maternal chorioamnionitis, hypovolemia secondary to placental abruption or a tight nuchal cord, neonatal sepsis and necrotizing enterocolitis (NEC) [10].
Maagement Strateies for Nonatal Hypotension
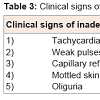
Volume expansion: Cautious volume administration of 10-20 ml/kg of a normal saline bolus is a reasonable approach in the initial management of hypotension in infants showing clinical evidence of hypovolemia and inadequate perfusion (Table 3) [6,11]. Since hypovolemia is a less common cause of hypotension in preterm infants, their response to volume expansion may be suboptimal [6]. Overzealous administration of fluids to preterm infants may be harmful. In a case-control study, Van Marter et al. found an association between excessive fluid administration and bronchopulmonary dysplasia [12]. Given the fact that hypovolemia is infrequent in hypotensive preterm babies, it would be prudent to recommend that no more than one fluid bolus between 10 and 20 mL/kg be used for treatment of hypotension unless there is evidence of significant fluid losses and/or hypovolemia [12]. Other conditions should also be considered, i.e. congenital heart disease, as further volume expansion may be detrimental in these patients as well [11]. Volume expansion in these cases may lead to fluid overload, signs of pulmonary edema and worsening of the clinical course.Dopamine: Dopamine is a naturally occurring precursor of norepinephrine, and 50% of its effects are due to the release of stored norepinephrine from terminal nerve endings [13]. Dopamine increases myocardial contractility and after load through dose dependent actions [6]. At low dosages, dopamine (0.5-2 ug/kg/min) dilates renal and splanchnic blood vessels leading to increased blood flow [6]. Medium dosages (2-10 ug/kg/min) predominantly increase the myocardial contractility by stimulation of β1 receptors [6]. High dosages (>10 ug/kg/min) increase peripheral vascular resistance by stimulation of α1 receptors [6]. Typically, medium or high dose dopamine is suggested for the treatment of hypotension in neonates.
Dobutamine: Dobutamine (2-15 ug/kg/min) increases cardiac output by augmenting stroke volume [14,15] and decreases the peripheral vascular resistance via stimulation of peripheral β2 receptors [16]. In addition, dobutamine increases myocardial contractility by stimulating myocardial β1 receptors. The major indication of dobutamine usage is in the treatment of hypotension in asphyxiated preterm infants with myocardial dysfunction [6].
A recent article by Gupta and Donn addresses the effectiveness of dopamine and dobutamine as first-line treatments of neonatal hypotension [13]. Proponents of dopamine suggest that it brings about a faster and more effective increase in blood pressure secondary to its potent vasoconstrictor effects especially at high doses. On the other hand, opponents suggest that tissue perfusion could be compromised by such intense vasoconstriction. The Cochrane Meta-Analysis [17] and the European Consensus Guidelines [18] both suggest that dopamine is more effective than dobutamine. Osborn et al. showed a greater increase in superior vena caval flow with dobutamine than with dopamine in term infants [19]. However, the effect of dobutamine on the blood flow in cerebral blood vessels or superior vena cava in preterm infants has not been evaluated. Filippi et al. showed that dopamine produced reversible reductions in thyroid stimulating hormone (TSH), T4 and prolactin, which may further lower blood pressure, while dobutamine did not show such effects [20]. Unfortunately, without larger, multi-centered trials comparing dopamine with dobutamine, no consensus on which agent is best for the treatment of neonatal hypotension can be reached.
Epinephrine and norepinephrine: Both epinephrine (0.1-0.3ug/kg/min) [21] and norepinephrine (0.02-0.1 ug/kg/min) [21] are used in the treatment of hypotension in preterm neonates who do not respond to high-dose dopamine and/ordobutamine [6,21]. Stimulation of α1 receptors causes vasoconstriction and an increase in systemic vascular resistance [6]. β1 receptors are cardio-selective and their stimulation causes increased myocardial contractility [6]. On the other hand, stimulation of peripheral β2 receptors causes vascular smooth muscle relaxation and broncho-dilatation [6]. Both epinephrine and norepinephrine induce similar increases in myocardial contractility and heart rate by stimulating β1 receptors [6]. Norepinephrine is a more potent vasoconstrictor as it predominantly stimulates α1 receptors.
In clinical practice, epinephrine administered at low dosages stimulates β receptors, leading to positive inotropic effects. However, an elevation in blood pressure may not be seen until epinephrine infusion is given at higher doses, which will also stimulate the α receptors in the peripheral vasculature. Although there is a lack of randomized clinical trials concerning epinephrine administration, some published data suggest that simultaneous infusions of epinephrine and dopamine increases blood pressure and urine output in preterm, hypotensive neonates [22]. Though epinephrine is widely used in neonatal resuscitation and blood pressure management, there are concerns regarding its safety. Current studies in adults suggest that high dosages of epinephrine may alter mesenteric blood flow to the bowel. It is unclear if the adverse effects of epinephrine observed in the adult population translate to the neonatal population. However, epinephrine and norepinephrine remain vital agents in the treatment of neonatal hypotension [23].
Vasopressin: Vasopressin (0.018-0.12 units/kg/h) [21] is an anti-diuretic hormone analogue that acts as a pure peripheral vasoconstrictor via stimulation of V1 receptors [21]. It has recently been suggested that vasopressin may be as effective as dopamine as an initial therapy for hypotension in extremely low birth weight (ELBW) infants [24]. The largest study to date was conducted by Choong et al. This multicenter, double blind, randomized controlled trial included 65 children ages 1 month-17 years and evaluated the safety and efficacy of vasopressin as an agent in the management of pediatric vasodilatory shock. This trial determined that lowdose vasopressin did not demonstrate any beneficial effects [25]. Vasopressin and its analogues may prove promising for use in the treatment of hypotension in neonates with additional data; however it is not currently recognized as a standard of care. Additional research is needed to determine appropriate timing and length of treatment, efficacy, and side effects.
Milrinone: Milrinone is a phosphodiesterase-III inhibitor that increases myocardial contractility and causes peripheral vasodilatation. Milrinone (0.5-0.75 ug/kg/min) may be used as a rescue treatment for hypotension in infants even though its mechanism of action remainsunclear [21]. Milrinone increases cardiac index, stroke volume and oxygen delivery [26]. It should be used carefully in infants with hypotension as its peripheral vasodilatory action may further lower the blood pressure [26]. Milrinone also has vasodilator effects on the pulmonary vasculature through a cyclic adenosine monophosphate-mediated signaling pathway. This effect is synergistic with the administration of inhaled nitric oxide (iNO) [23]. In a case series with 7 infants, milrinone improved oxygenation index and achieved a reduction in iNO dose within 24 hours of its administration. There was also a reduction in pulmonary pressure assessed by echocardiogram and a concomitant increase in pulmonary venous return [24]. Another study showed that milrinone led to improved oxygenation in patients with suboptimal response to iNO. Serial echocardiograms performed as a part of the study demonstrated lower pulmonary artery pressures, improved right and left ventricular output and reduced right to left shunting [27]. A Cochrane Database systemic review assessing the role of milrinone in the treatment of persistent pulmonary hypertension of the newborn (PPHN) determined that the efficacy and safety of milrinone in the treatment of PPHN was not clearly determined and recommended that its use be restricted and judicious [28]. Milrinone, used prophylactically (75-μg/kg bolus followed by a 0.75- μg/kg per min infusion), has also been shown to reduce the risk of low cardiac output syndrome after pediatric congenital heart surgery. Although hypotension, thrombocytopenia, and arrhythmias have been reported side effects in adult patients, these events occurred less frequently in pediatric patients [29]. Milrinone has diverse and complex effects on systemic and pulmonary hemodynamics. We do not recommend milrinone as a first choice agent for the treatment of neonatal hypotension as further review is necessary to determine its role in PPHN.
Though there are a myriad of agents that maybe used in neonatal hypotension, this review article will focus mainly on hydrocortisone.
Hydrocortisone
Pharmacology: Hydrocortisone is an identical molecule to the glucocorticoid hormone cortisol, which is secreted by the adrenal cortex [5,30]. Cortisol’s secretion is regulated by adrenocorticotrophic hormone (ACTH) released from the anterior pituitary gland, and by corticotrophin releasing hormone (CRH), which is released by the neurons of the hypothalamus [30]. The release of cortisol is regulated by a negative feedback mechanism (Figure 1). The human stress response involves a complex signaling pathway among neurons and somatic cells resulting in the release of two peptide hormones, CRH and arginine-vasopressin (AVP), from the hypothalamus. The roles of the two peptide hormones, CRH and AVP, have been widely studied [31]. Stress stimulates the release of both CRH and AVP from neurons in the hypothalamus. CRH is then transported to the anterior pituitary gland where it stimulates the secretion of ACTH. ACTH then stimulates the secretion of corticosteroids, including cortisol. Together, CRH and AVP are involved in the activation of the hypothalamic-pituitary-adrenal (HPA) axis; a complex system of feedback interactions reviewed in Figure 2 [31]. The various actions of hydrocortisone are summarized in Table 4.Figure 2: The mechanism of genomic and non-genomic pathways of corticosteroids.Genomic pathway: The corticosteroid (CC) molecule enters the cell cytoplasm and binds with a glucocorticoid receptor (GR). The complex then diffuses to the nucleus, binds to specific DNA sequences, and increases the synthesis of messenger RNA (mRNA) leading to increased protein synthesis.Non-genomic pathway: The corticosteroid (CC) molecule binds to a receptor (R) on the cell membrane. This complex activates protein kinases leading to increased expression of second messengers such as cyclic adenosine monophosphate (cAMP).
Mechanisms of action of hydrocortisone
Corticosteroids mediate their actions via parallel genomic and non-genomic pathways (Figure 2).
Genomic pathway: In the genomic pathway, hydrocortisone utilizes transactivation to produce cellular proteins. Transactivation begins as hydrocortisone diffuses across target cell membranes and binds with corticosteroid receptors located in the cytoplasm [31]. The hydrocortisone-receptor complex is then transported to the nucleus, where it binds with a specific DNA sequence known as glucocorticoid response elements (GRE) located in the promoter region of the target genes. This new complex then regulates the transcription of mRNA molecules and gene expression [32]. This process leads to the production of new proteins that have a wide range of effects [33]. Specific examples include the anti-inflammatory proteins lipocortin-1 and secretory leukoprotease inhibitor-1, along with gluconeogenic proteins such as glucose-6-phosphate and tyrosine amino-transferase [34]. Transactivation is a slow process that may take hours or even days before significant quantities of new proteins are produced [32]. As a result, most of the genomic effects of hydrocortisone are delayed [30]. Therefore, hydrocortisone is not recommended for urgent control of blood pressure. Consideration should be given to early administration of hydrocortisone given its delayed onset of action.
Non-genomic pathway: The non-genomic pathway was first reported as a separate mechanism in 1942 when it was observed that certain corticosteroids had an anesthetic effect within minutes of administration [32]. This pathway is predominantly mediated by receptors located on the cell membrane, which serve to generate cyclic adenosine monophosphate or protein kinases that act as second messengers in the cytoplasm [32]. In contrast to the genomic pathway, this alternative route leads to more rapid effects that occur within minutes of onset [32]. Even though these effects are relatively rapid in onset, they are still slower than volume expansion or vasopressors. The effects mediated by the non-genomic pathway are usually short lived (< 60 minutes) and its mechanism is still not clearly understood [32]. Therefore, hydrocortisone is not recommended to urgently control hypotension in neonates.
The mechanisms of hydrocortisone in the treatment of hypotension
During the course of a critical illness, downregulation of the adrenergic receptors [6,35] leads to a gradual desensitization of the cardiovascular system to the effects of catecholamines [6]. In addition, a relative or absolute adrenal insufficiency may contribute to hypotension that is resistant to vasopressor treatment [6,36,37]. In this setting, hydrocortisone works through the following mechanisms:
a) Induction of the expression of cardiovascular adrenergic receptors (genomic effect) [6].
b) Inhibition of the expression of inducible nitric oxide synthase and vasodilatory prostaglandin action (genomic effect) [38].
c) Inhibition of catecholamine metabolism, reuptake and therelease of vasoactive factors (non-genomic effect) [5,6,38].
d) Increase in the intracellular calcium concentration leading to enhanced myocardial and vascular responsiveness tocatecholamines (non-genomic effect) [6,38].
A special consideration in preterm infants
In some very low birth weight (VLBW) infants, hypotension is refractory to both volume expansion and vasopressor treatment [39]. A study by Ng et al. showed an increase in plasma ACTH with the use of inotropes and volume expanders in VLBW infants with hypotension. In addition, a low serum cortisol was also found in these patients [39]. Due to this finding, the authors hypothesized that the adrenal cortex is the primary site of dysfunction in this patient population; not the hypothalamus or pituitary gland. Overall, these findings suggested a normal response of the pituitary gland to hypotension, while the adrenal glands were ‘transiently’ unable to maintain cortisol secretion in the immediate postnatal period. This phenomenon is known as transient adrenocortical insufficiency of prematurity (TAP) [39]. Transient adrenocortical insufficiency is characterized by the following:
a) Commonly presents in the 1st week of life in extremely preterm infants [39].
b) Resistant to conventional volume expansion and inotropic support [39].
c) Prompt and efficient response to hydrocortisone administration [40-43].
d) Adrenal recovery and normalization of circulating cortisol levels by 2 weeks of life [40-43].
The transient nature of TAP may be attributed to the adrenocortical immaturity in preterm infants leading to a deficiency of intermediate enzymes of cortisol synthesis (21-hydroxylase and 11β-hydroxylase) [44-47]. However, TAP may be prolonged in some extremely low birth weight infants [39].
Indications and dosing
Hydrocortisone is recommended in the treatment of hypotensive neonates if volume resuscitation with isotonic saline and dopamine treatment (10 mcg/kg/min) is unsuccessful [5]. The recommended starting dose is 1-2 mg/kg every 12 hours intravenously [43,44]. The dosing intervalis 6-8 hours for infants >35 weeks of gestation and 8-12 hours for infants < 35 weeks of gestation [5]. Parameters of improvement within 24 hours of administration of hydrocortisone include: a) improvement in blood pressure, b) improvement in urine output and c) ability to reduce vasopressor support [5]. When all of these parameters are achieved, reducing the dose to 0.5 mg/kg/dose is recommended [5]. If the duration of treatment is three days or less, hydrocortisone may be discontinued without tapering as the HPA axis is not suppressed [48,49].
Side effects
Short Term (≤3 days): These include hypokalemia, esophagitis, impaired wound healing, hypertension and hyperglycemia [5]. Gastric bleeding and gut perforation are possible side effects if hydrocortisone is administered in combination with non-steroidal anti-inflammatory drugs such as in domethacin, which is commonly used to induce PDA closure [50]. Hydrocortisone is contraindicated in infants with systemic fungal infections, as secondary hyperglycemia provides an enriched medium for candida growth [51]. Hydrocortisone also reduces phagocytosis and the number of functional lymphocytes leading to decreased immune competency [52].
Long Term (≥2 weeks): Prolonged use may be defined as constant administration for 2 weeks or intermittent administration for a total of 3 weeks during a 6 month period or longer [53]. Patients exposed to long-term hydrocortisone therapy are likely to develop adrenal insufficiency secondary to hypothalamic-pituitary-adrenal axis suppression. Although there is evidence of neurodevelopmental impairment after prolonged use of hydrocortisone in ELBW infants (≤1000 gm) [54], the use of low dose hydrocortisone (1 mg/kg/day for 12 days followed by 0.5 mg/kg/day for 3 days) for the treatment of adrenal insufficiency in extremely low birth weight infants has not been found to be associated with an increased incidence of cerebral palsy [55].
Clinical outcomes of infants treated with hydrocortisone
Various studies have shown that hydrocortisone improves the hemodynamic status in neonates. The clinical effects of hydrocortisone include:
a) Increase in arterial blood pressure [56].
b) Ability to reduce dose of inotropes-in one study, authors noted significant and progressive reduction in total doses of dopamine and dobutamine at 6 (p=0.017), 12 (p< 0.0001) and 24 (p< 0.0001) hours after starting hydrocortisone [56].
c) Improvement in diuresis [56].
d) Ability to reduce dopamine dose by 62% 24 hours after starting hydrocortisone (21 ug/kg/min to 8 ug/kg/min) and by 72% 48 hours after starting hydrocortisone (21 ug/kg/min to 6 ug/kg/min) [57].
Who is likely to benefit from hydrocortisone?
Preterm infants: Cortisol values vary inversely with gestational age [58]. The reference range for cortisol values in preterm infants within the first 2 weeks of life is reported as 165±25 nmol/l [59]. Since the hypothalamic-pituitary axis is immature in preterm infants, a normal cortisol level during periods of stress may indicate relative adrenal insufficiency [60]. Sick preterm infants with low/normal cortisol levels, or those not responding to vasopressor therapy, may benefit from the use of hydrocortisone [60]. In addition, a 2010 meta-analysis suggested that hydrocortisone administration significantly increases blood pressure in hypotensive, preterm infants who exhibit vasopressor resistance [48]. However, it is still unclear as to whether hydrocortisone’s beneficial cardiovascular actions lead to clinically relevant medium to long-term outcomes. Although prophylactic hydrocortisone therapy was not examined in this metaanalysis, hydrocortisone’s efficacy for increasing blood pressure and decreasing vasopressor dependence in hypotensive, preterm infants suggests that prophylactic use of hydrocortisone may be beneficial in preventing hypotension in the preterm neonates [61].
Term infants: The published reference value for cortisol in term infants within 2 weeks of life is171±27 nmol/l [59]. However, a careful consideration of the patient’s clinical picture remains vital as different studies have reported a range of reference values similar to values seen in preterm infants. In a study of hydrocortisone use in vasopressor-resistant, hypotensive, term (≥37 weeks) infants, Fernandez et al. found that a majority of critically ill, term infants had low cortisol concentrations consistent with relative adrenal insufficiency [49]. In addition, these infants displayed low endogenous ACTH concentration, while exhibiting a normal response to low dose, exogenous ACTH. Taken together, these findings showed that a secondary insufficiency due to inadequate stimulation of the adrenal gland, not an insufficiency of the adrenal gland itself, was responsible for these patients’ symptoms. One possible explanation for this inadequate stimulation may be due to withdrawal of placental CRH during delivery. This suggests that low dose hydrocortisone replacement may be a more appropriate therapy than vasopressors in these infants. In addition, infants with cortisol levels < 15 mcg/dl were more responsive to hydrocortisone therapy. This was proven by improvement in blood pressure, decreased heart rate and the ability to wean vasopressor and volume support [49,60].
The issue of ACTH stimulation testing
Based on available studies, a dose of 1 μg/kg of ACTH is the most effective dose in discerning adrenal insufficiency in neonates [62,63]. Recently, the American College of Critical Care Medicine proposed the term “critical illness related corticosteroid insufficiency” (CIRCI) to replace the former use of “relative adrenal insufficiency”. CIRCI is defined by an inadequate increase in corticosteroid activity in response to the severity of the patient’s illness. CIRCI is diagnosed by obtaining a cortisol level of less than 9 μg/dl (248 nmol/l) after administering a dose of ACTH [59]. At this time, there are no specific recommendations regarding whether ACTH stimulation testing is warranted if cortisol levels do not normalize by 2 weeks of life. The clinical picture, as well as the signs and symptoms of cortisol deficiency, should be considered prior to obtaining this test.
Role of dexamethasone in neonatal hypotension
A double-blinded placebo-controlled study showed that a single dose of 0.25 mg dexamethasone improved severe hypotension in preterm infants unresponsive to volume expansion and dopamine infusion within 12 hours of administration [64]. As discussed previously, a relative cortisol deficiency may be the reason for severe hypotension in premature and severely ill preterm neonates. The rapid and sustained effect of dexamethasone observed in this study corresponded with a relative adrenal insufficiency. Although the study did not evaluate the safety of dexamethasone administration, it showed that dexamethasone might be as effective as hydrocortisone in treating hypotensive preterm infants who have not responded to dopamine. The dose of dexamethasone corresponded to approximately 60 mg/m2 of hydrocortisone. In this study, dexamethasone administration was limited to a single dose, whereas all other available studies included repeated dexamethasone doses [65,66]. Single-dose administration may be preferable because of the reduced incidence of undesired side effects.
Conclusion
Multiple agents are used in the management of neonatal hypotension. The majority of the pharmacologic effects of these agents is dose dependent and requires careful attention to dosing in order to obtain desired outcomes. Current data supports volume expansion as the first-line treatment of hypotension, followed by the administration of dopamine. More studies are needed to establish the safety and efficacy of agents such as milrinone and vasopressin in the management of hypotension. Hydrocortisone should be used if standard volume expansion and pressor support is not effective. Hydrocortisone is not recommended for urgent control of hypotension. It should be discontinued as soon as clinical parameters permit to prevent suppression of the hypothalamic-pituitary-adrenal axis. Close monitoring for potential side effects and complications of hydrocortisone exposure should be implemented.References
- Engle WD (2008) Definition of normal blood pressure range: the elusive target. In: Kleinmann CS, Seri I (Eds). Hemodynamics and cardiology: Neonatology questions and controversies. Saunders/Elsevier Co: Philadelphia, pp. 49-66.
- Cayabyab R, McLean CW, Seri I (2009) Definition of hypotension and assessment of hemodynamics in the preterm neonate. J Perinatol 29: 558-562.
- Evans N, Seri I (2005) Cardiovascular compromise in the newborn infant. In: Taeusch HW, Ballard RA, Gleason CA (Eds). Avery’s Disease of the Newborn, 8th edition. Elsevier Sanders, USA, pp. 398-409.
- Shead SL (2015) Pathophysiology of the cardiovascular system and neonatal hypotension. Neonatal Netw 34: 31-38.
- Johnson PJ (2015) Hydrocortisone for treatment of hypotension in the newborn. Neonatal Netw 34: 46-50.
- Seri I (2001) Circulatory support of the sick preterm infant. Semin Neonatol 6: 85-95.
- Patural H, Barthelemy JC, Pichot V, Mazzochi C, Teyssier G, et al. (2004) Birth prematurity determines prolonged autonomic nervous system immaturity. Clin Auton Res 14: 391-395.
- Weindling AM, Subhedar NV (2007) The definition of hypotension in very low-birthweight infants during the immediate neonatal period. Neoreviews 8: e32-e43.
- Noori S, Seri I (2008) Etiology pathophysiology and phases of neonatal shock. In: Polin RA (Ed). Neonatology questions and controversies: hemodynamics and cardiology. Saunders/Elsevier Co: Philadelphia, pp. 3-18.
- Seri I (2006) Management of hypotension and low systemic blood flow in very low birth weight neonate during the first postnatal week. J Perinatol 26 Suppl 1: S8-S13.
- Turner DA, Cheifetz IM (2015) Shock. In: Kliegman RM, Stanton BF, St Geme III JW, Schor NF, Behrman RE Nelson (Eds). Textbook of Pediatrics, (20th edn). Elsevier 1: 516-527.
- Ibrahim CP (2008) Hypotension in preterm infants. Indian Pediatr 45: 285-294.
- Gupta S, Donn SM (2014) Neonatal hypotension- dopamine or dobutamine? Semin Fetal Neonatal Med 19: 54-59.
- Rozé JC, Tohier C, Maingueneau C, Lefèvre M, Mouzard A (1993) Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch Dis Child 69: 59-63.
- Bhatt-Mehta V, Nahat MC (1989) Dopamine and dobutamine in pediatric therapy. Pharmacotherapy 9: 303-314.
- Ruffolo RR Jr (1987) The pharmacology of dobutamine. Am J Med Sci 294: 244-248.
- Subhedar NV, Shaw NJ (2003) Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database Syst Rev: CD001242.
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, et al. (2013) European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants--2013 update. Neonatology 103: 353-368.
- Osborn D, Evans N, Kluckow M (2002) Randomised trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr 140: 183-191.
- Filippi L, Pezzati M, Poggi C, Rossi S, Cecchi A, et al. (2007) Dopamine versus dobutamine in very low birthweight infants: endocrine effects. Arch Dis Child Fetal Neonatal Ed 92: F367-F371.
- Turner MA, Baines P (2011) Which inotrope and when in neonatal and pediatric intensive care? Arch Dis Child Educ Pract Ed 96: 216-222.
- Schmaltz C (2009) Hypotension and shock in the preterm neonate. Adv Neonatal Care 9: 156-162.
- James AT, Bee C, Corcoran JD, McNamara PJ, Franklin O, et al. (2015) Treatment of premature infants with pulmonary hypertension and right ventricular dysfunction with milrinone: a case series. J Perinatol 35: 268-273.
- Rios DR, Kaiser JR (2015) Vasopressin versus dopamine for treatment of hypotension in extremely low birth weight infants: a randomized, blinded pilot study. J Pediatr 166: 850-855.
- Choong K, Bohn D, Fraser DD, Gaboury I, Hutchison JS, et al. (2009) Vasopressin in pediatric vasodilatory shock: a multicenter randomized controlled trial. Am J Respir Crit Care Med 180: 632-639.
- Caresta E, Papoff P, Valentini SB, Mancuso M, Cichetti R, et al. (2011) What’s new in the treatment of neonatal shock. J Matern Fetal Neonatal Med 24S: S17-S19.
- McNamara PJ, Shivananda SP, Sahni M, Freeman D, Taddio A (2013) Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatr Crit Care Med 14: 74-84.
- Bassler D, Kreutzer K, McNamara P, Kirpalani H (2010) Milrinone for persistent pulmonary hypertension of the newborn. Cochrane Database Syst Rev: CD007802.
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, et al. (2003) Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 107: 996-1002.
- Brunton LL, Lazo JS, Parker KL (2005) Goodman and Gilman’s the pharmacological basis of therapeutics, Eleventh edition. McGraw Hill Professional, pp. 1587-1612.
- Randall M (2011) The physiology of stress: Cortisol and the hypothalamic-pituitary-adrenal axis. Dartmouth Undergraduate J Sci.
- Rodrigo GJ (2006) Inhaled corticosteroids in the treatment of asthma exacerbations: essential concepts. Arch Bronconeumpl 42: 533-540.
- Evollo JR, Cidlowski JA (2009) Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci 1179: 167-178.
- Newton R, Holden NS (2007) Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol 72: 799-809.
- Hausdorff WP, Caron MG, Lefkowitz RJ (1990) Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J 4: 2881-2890.
- Korte C, Styne D, Merritt TA, Mayes D, Wertz A, et al. (1996) Adrenocortical function in the very low birth weight infant: improved testing sensitivity and association with neonatal outcome. J Pediatr 128: 257-263.
- Watterberg KL, Gerdes JS, Gifford KL, Lin HM (1999) Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics 104: 1258-1263.
- Rios DR, Moffet BS, Kaiser JR (2014) Trends in pharmacotherapy for neonatal hypotension. J Pediatr 165: 697-701.
- Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, et al. (2004) Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birth weight infants. Arch Dis Child Fetal Neonatal Ed 89: F119-F126.
- Helbock HJ, Insoft RM, Conte FA (1993) Glucocorticoid-responsive hypotension in extremely low birth weight newborns. Pediatrics 92: 715-716.
- Fauser A, Pohlandt F, Bartmann P, Gortner L (1993) Rapid increase of blood pressure in extremely low birth weight infants after a single dose of dexamethasone. Eur J Pediatr 152: 354-356.
- Gaissmaier RE, Pohlandt F (1999) Single-dose dexamethasone treatment of hypotension in preterm infants. J Pediatr 134: 701-705.
- Ng PC, Lam CW, Fok TF, Lee CH, Ma KC, et al. (2001) Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed 84: F122-F124.
- Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA (1994) Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab 78: 266-270.
- Lee MM, Rajagopalan L, Berg GJ, Moshang T Jr (1989) Serum adrenal steroid concentrations in premature infants. J Clin Endocrinol Metab 69: 1133-1136.
- Huysman MW, Hokken-Koelega AC, De Ridder MA, Sauer PJ (2000) Adrenal function in sick very preterm infants. Pediatr Res 48: 629-633.
- Watterberg KL, Gerdes JS, Cook KL (2001) Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr Res 50: 190-195.
- Higgins S, Friedlich P, Seri I (2010) Hydrocortisone for hypotension and vasopressor dependence in preterm neonates: a meta-analysis. J Perinatol 30: 373-378.
- Fernandez EF, Montman R, Watterberg KL (2008) ACTH and cortisol response to critical illness in term and late preterm newborns. J Perinatol 28: 797-802.
- Stokowski LA (2004) Controversies in using steroids: from fetus to newborn. Medscape Multispecialty.
- Rowen JL, Atkins JT, Levy ML, Baer SC, Baker CJ (1995) Invasive fungal dermatitis in the < or = 1000-gram neonate. Pediatrics 95: 682-687.
- Pera A, Byun A, Gribar S, Schwartz R, Kumar D, et al. (2002) Dexamethasone therapy and Candida sepsis in neonates less than 1250 grams. J Perinatol 22: 204-208.
- Ahmet A, Kim H, Spier S (2011) Adrenal suppression: A practical guide to the screening and management of this under-recognized complication of inhaled corticosteroid therapy. Allergy Asthma Clin Immunol 7: 13.
- Patra K, Greene MM, Silvestri JM (2015) Neurodevelopmental impact of hydrocortisone exposure in extremely low birth weight infants: outcomes at 1 and 2 years. J Perinatol 35: 77-81.
- Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, et al. (2007) Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 120: 40-48.
- Baker CF, Barks JD, Engmann C, Vazquez DM, Neal CR Jr, et al. (2008) Hydrocortisone administration for the treatment of refractory hypotension in critically ill newborns. J Periantol 28: 412-419.
- Noori S, Friedlich P, Wong P, Ebrahimi M, Siassi B, et al. (2006) Hemodynamic changes after low-dosage hydrocortisone administration in vasopressor-treated preterm and term neonates. Pediatrics 118: 1456-1466.
- Scott SM, Watterberg KL (1995) Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr Res 37: 112-116.
- Quintos JB, Boney CM (2010) Transient adrenal insufficiency in the premature newborn. Curr Opin Endocrinol Diabetes Obes 17: 8-12.
- Aucott SW (2005) Hypotension in the newborn: who needs hydrocortisone? J Perinatol 25: 77-78.
- Efird MM, Heerens AT, Gordon PV, Bose CL, Young DA (2005) A randomized-controlled trial of prophylactic hydrocortisone supplementation for the prevention of hypotension in extremely low birth weight infants. J Perinatol 25: 119-124.
- Watterberg KL, Shaffer ML, Garland JS, Thilo EH, Mammel MC, et al. (2005) Effect of dose on response to adrenocorticotropin in extremely low birth weight infants. J Clin Endocrinol Metab 90: 6380-6385.
- Langer M, Modi BP, Agus M (2006) Adrenal insufficiency in the critically ill neonate and child. Curr Opin Pediatr 18: 448-453.
- Gaissmaier RE, Pohlandt F (1999) Single-dose dexamethasone treatment of hypotension in preterm infants. J pediatr 134: 701-705.
- Derleth DP (1992) Extraordinary response to dexamethasone therapy for hypotension in a premature newborn: a case report. Pediatr Res 31(4 Part 2): 200a.
- Derleth DP (1994) Blood pressure in low birth weight infants after dexamethasone. Eur J Pediatr 153: 211.