Journal of Plant Biology & Soil Health
Download PDF
Research Article
*Address for Correspondence: Keithellakpam Sanatombi, Department of Biotechnology, Manipur University, Imphal, Manipur 795003, India, Tel: 03852435233; Fax: 03852435233; E-mail: ksanatombi@rediffmail.com
Screening and Quantification of Artemisinin and Phytochemicals Content in Artemisia nilagarica (C.B. Clarke) Pamp.
Mayengbam Nganthoi, Jina Heikrujam, Khaidem Chanu Kabita and Keithellakpam Sanatombi*
- Department of Biotechnology, Manipur University, Imphal, Manipur 795003, India
*Address for Correspondence: Keithellakpam Sanatombi, Department of Biotechnology, Manipur University, Imphal, Manipur 795003, India, Tel: 03852435233; Fax: 03852435233; E-mail: ksanatombi@rediffmail.com
Abstract
The present study reports, for the first time, the quantification of secondary metabolites including artemisinin in field grown plant samples and in vitro regenerated plantlets and callus tissues of Artemisia nilagarica (C.B. Clarke) Pamp. The influence of different growth regulators on in vitro micropropagation and callus induction was investigated. Maximum shoot multiplication from shoot tip explants was observed in explants cultured on Murashige and Skoog medium supplemented with 1 mg/L naphthalene acetic acid in combination with 5 mg/L kinetin followed by medium supplemented with 1 mg/L naphthalene acetic acid in combination with 3 mg/L kinetin and the shoot buds also showed rooting in these media. Rooted plantlets were successfully established in the soil. Friable callus was induced from shoot tip explants of in vitro regenerated plantlets on medium supplemented with combination of 1 mg/L naphthalene acetic acid and 5 mg/L 6-benzylaminopurine. Secondary metabolites such as phenol, alkaloid, tannins, saponins, flavonoids, terpenoids, steroids, phlobatannins, chalcones and anthraquinones were found to be present in A. nilagarica. The highest amount of alkaloid, saponin, steroid and DPPH free radical scavenging activity was obtained in shoot tips, stems, roots and leaves respectively while the artemisinin content was higher in leaves of field grown plants than in in vitro regenerated plantlets and callus tissues.Keywords
Artemisia nilagarica (C.B. Clarke) Pamp; Micropropagation; Callus; Secondary metabolites; Artemisinin; SpectrophotometryAbbreviations
BAP: 6-Benzylaminopurine; DPPH: 2,2-Diphenyl-1-Picrylhydrazyl; IAA: Indole-3-Acetic Acid; IBA: Indole-3-Butyric Acid; Kin: Kinetin; MS: Murashige and Skoog medium; NAA: Naphthalene Acetic Acid; TLC: Thin Layer Chromatography; HPLC: High Performance Liquid ChromatographyIntroduction
Artemisia is one of the largest genera of the Asteraceae family and about 34 species including A. nilagarica (C.B. Clarke) Pamp. are found in India [1]. The genus Artemisia is an important medicinal plant and many of the species are economically important as a source of essential oils, secondary metabolites, medicines, food, forage, ornamentals or soil stabilizers in disturbed habitats [2]. Like A. annua, the most important source of the anti-malarial drug artemisinin, A. nilagarica has also been shown to have high potency against the malaria parasite, Plasmodium falciparum [3,4]. A. nilagarica is commonly known as ‘Laibakngou’ in Manipur and its leaf extracts are used by local healers for treatment of wounds on skin, mouth sores and also as a tonic, antiseptic, analgesic, stomachic and insect repellent or as anti-diabetic [5-8]. A. nilagarica has been listed in the threatened medicinal plant category [9] and the species is over-exploited for its diverse medicinal properties [10]. Therefore, there is an urgent need to develop an alternative means for increased production of the important secondary metabolites and other important components of the plant. Plants obtained through micropropagation and in vitro cultured cells provide an efficient method for production of useful secondary metabolites irrespective of growing seasons and also minimize the over-exploitation of the plants from nature. There have been few reports on micro-propagation and callus induction in A.nilagarica [9] and no study has been conducted to estimate the artemisinin production potential and quantification of secondary metabolites of in vitro cultures of the plant. Therefore, the present study was undertaken to comparatively explore the content in field grown plant samples and in in vitro regenerated plants and callus tissues of A. nilagarica.Materials and Methods
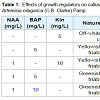
Micro-propagationShoot tips excised from field grown plants of A. nilagarica (C. B. Clarke) Pamp. were surface sterilized and cultured on MS medium (Murashige and Skoog, 1962) supplemented with 1 mg/L naphthalene acetic acid (NAA) in combination with 3 mg/L or 5 mg/L kinetin (Kin) for shoot bud multiplication. The multiplied shoot buds were transferred to the same multiplication medium for further multiplication and rhizogenesis. The rooted plantlets obtained after two months were then transferred to plastic cups containing sand:soil mixture (1:1) which were frequently watered and kept covered with perforated clear polythene bags to maintain humidity. The plants were then transferred to field condition after 2 months.
Callus induction
For callus induction, young shoot tips excised from in vitro multiplied plantlets were cultured on MS medium supplemented with 5 mg/L 6-benzylaminopurine (BAP) or Kin alone or 1 mg/L NAA in combination with 5 and 10 mg/L BAP or 10 mg/L Kin.All cultures were incubated in a growth chamber with temperature maintained at 25 ± 1 °C and a 16-h photoperiod. Each treatment for shoot induction, root induction and callus induction had ten replicates and the experiments were repeated thrice.
Phytochemical screening
The coarsely powdered ex vitro shoot tips, leaves, stems, roots, in vitro leaves and callus of A. nilagarica were extracted with different solvents, such as chloroform, ethyl acetate, ethanol, distilled water and hexane. The herb to solvent ratio was kept 1:10 to ensure complete extraction. The plant material was extracted by cold maceration for 72 hours with intermittent agitation. After incubation, the extracts were filtered through Whatman filter paper and the extracts were stored at 4 °C till further use.
Qualitative estimation of secondary metabolites
The extracts obtained were subjected to Mayer’s test, Dragendorff’s test, Salkowski test, ammonia test, and test for phenols, tannins, saponins, flavonoids, steroids (Glycosides), phlobatannins and anthraquinones as per the methods given by Harborne [11].
Quantitative estimation of secondary metabolites
For quantitative estimation of secondary metabolites, the extracts obtained were subjected to alkaline precipitation gravimetric method for alkaloid determination, double extraction gravimetric method for saponin determination and steroid determination as per the methods given by Harborne [11]. Powdered samples were extracted in methanol with 1:10 sample to solvent ratio and the methanol extracts were used for the quantitative analysis of secondary metabolites and estimation of DPPH free radical scavenging activity. The total phenol, flavonoids, tannin contents and total antioxidant potential in the methanol extracts were determined using Folin-Ciocalteau reagent method [12], aluminum chloride method [12], Folin-Dennis Spectrophotometric method [13] and DPPH analysis method of Shimada et al. respectively [14].
Artemisinin estimation
Stock solution (1 mg/mL) of standard artemisinin (from Sigma Aldrich, New Delhi) was prepared in 95% ethanol. Serial dilution was done for standard curve preparation. Powdered dried samples of young leaves from field grown plants, leaves from six-week old in vitro propagated plantlets and callus tissues were used for extraction of artemisinin by using n-hexane. The concentrations of artemisinin present in the samples were determined by UV-Vis Spectrophotometry method specified for artemisinin determination by Tarsisius et al. [15].
Results and Discussion
Multiple shoot buds proliferated from the shoot-tip explants cultured on bud induction medium containing 1 mg/L NAA in combination with 5 mg/L Kin after four weeks of culture and a maximum of 15 shoot buds were obtained per explants (Figure 1a). Earlier, medium supplemented with 1 mg/L BAP in combination with 0.3 or 1 mg/L NAA was found to be effective for micropropagation of A. nilagarica through indirect organogenesis from callus [9]. However, in the present study, medium supplemented with combinations of Kin with NAA was more effective than combinations of BAP with NAA and hence BAP was not used for shoot multiplication. The shoot buds cultured on medium containing 1 mg/L NAA in combination with 5 mg/L Kin also showed the induction of rooting and further multiplication was achieved by sub culturing the buds on these media every four weeks (Figure 1b ). The in vitro regenerated plantlets showed 70-80% survival during transplantation (Figure 1c). The shoot tip explants produced friable callus with the best morphological and growth characteristics suitable for subculture was obtained on medium supplemented with 1 mg/L NAA and 5 mg/L BAP with 100% response (Table 1 and Figure 1d). Similar effectiveness of the combination of NAA and BAP in callus induction in A. nilagarica has been reported earlier [9].Figure 1: In vitro culture of Artemisia nilagarica (C.B. Clarke) Pamp., (a) Proliferation of shoot buds from shoot tip explants cultured on medium supplemented with 1 mg/L NAA and 5 mg/L Kin; (b) Rooting from shoot tip explants cultured on medium supplemented with 1 mg/L NAA and 5 mg/L Kin; (c) Transplanted plantlet and (d) Callus induced from shoot tip explants of A. nilagarica cultured on MS medium supplemented with 1 mg/L NAA and 5 mg/L BAP.
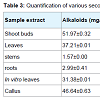
Qualitative screening of secondary metabolites revealed the presence of medicinally active constituents like phenol, alkaloid, tannins, saponins, flavonoids, terpenoids, steroids, phlobatannins, chalcones and anthraquinones in extracts of shoots, leaves, stems, roots, in vitro leaves and callus of A. nilagarica obtained by using different solvents (Table 2). Similar findings have also been reported earlier [4,16-18] except for some minor differences in the presence or absence of the metabolites in the different extracts. The highest alkaloid content was obtained in the methanol extracts of shoot buds (51.97 mg/g) followed by callus (46.64 mg/g), young leaves (37.21 mg/g), in vitro leaves (31.38 mg/g), stems (1.57 mg/g) and roots (2.99 mg/g) (Table 3). The saponin content was highest in stems (32.26 mg/g) whereas the steroid content was highest in the roots (21.96 mg/g). The phenol, flavonoids and tannins contents of the methanol extracts were low and it was less than 5 mg/g in all the samples. The highest DPPH free radical scavenging activity in terms of % inhibition of DPPH was shown by leaf extracts (65%) followed by shoot bud extracts (64%).
There is only one earlier report on artemisinin content analysis in A. nilagarica and the content was so low that it could not be quantified significantly by HPLC method [19]. However, in the present study, even though the artemisinin concentration was low, it was quantified in leaves of field grown plant (0.14%), in vitro leaves (0.07%) and callus tissues (0.05%) of A. nilagarica. The artemisinin content in leaves of field grown plants was higher than in in vitro regenerated plantlets and callus tissue. Absorption maximum of artemisinin is obtained at 298 nm which was against 290 nm reported by Tarsisius et al. [15]. The regression equation calculated from the standard curve of artemisinin was Y = 0.128X + 0.358 (where, ‘Y’ is the absorbance and ‘X’ is the concentration) and R2 = 1.
Conclusion
The present study, thus, presents a preliminary report on the quality and quantity of various secondary metabolites including artemisinin and DPPH free radical scavenging activities in various explants of A. nilagarica. Further work is being carried out to optimize in vitro artemisinin production by cell suspension cultures of the plant.Acknowledgement
One of the authors (M. Nganthoi) acknowledges University Grants Commission (UGC), New Delhi, India, for providing Junior Research Fellowship (JRF) vide grant number 17-31/08(SA-I).References
- Suresh J, Mruthunjaya K, Paramakrishnan N, Naganandhini MN (2010) Determination of artemisinin in Artemisia arbotanum and Artemisia pallens by LC/MS method. Int J Curr Pharm Res 3: 49-52.
- Valles J, Torrell M, Garnatje T, Garcia-Jacas N, Vilatersanaand R, et al. (2003) Genus Artemisia and its allies, phylogeny of the sub-tribe Artemisiinae (Asteraceae, Anthemadea) based on nucleotide sequences of nuclear ribosomal DNA internal transcribed spacers (ITS). Plant Biol 5: 274-284.
- Chopra RN, Chopra IC, Handa KL, Kapoor LD (1994) Indigenous drug of India, (2ndedn). Academic Publication, Calcutta.
- Devmurari VP, Pandey S, Goyani MB, Jivani NP (2010) Phytochemical screening of ethanolic extract of Artemisia nilagarica. Int J Chem Sci 8: 2099-2104.
- Devi WI, Devi GS, Singh CB (2011) Traditional herbal medicine used for the treatment of diabetes in Manipur, India. Res J Pharm Biol Chem Sci 2: 709-715.
- Singh LW (2011) Traditional medicinal plants of Manipur as anti-diabetics. J Med Plants Res 5: 667-687.
- Lokho A (2012) The folk medicinal plants of the Mao Naga in Manipur, North East India. Int J Sci Res Pub 2: 1-8.
- Ningombam DS, Singh PK (2014) Ethnobotanical study of Phologacanthus thyrsiformis Nees: a conserved medicinal plant of Manipur, Northeast India. Int J Herb Med 1: 10-14.
- Ganesan CM, Paulsamy S (2011) Mass propagation of a threatened medicinal plant, Artemisia nilagarica (C.B. Clarke) Pampanini inhabiting high hills of Nilgiri, the Western Ghats. Indian J Fund Appl Life Sci 1: 14-21.
- Paulsamy S, Senthilkumar P, Shivashanmugam M (2008) Clonal multiplication strategies for medicinal plants inhabiting Nilgiri biosphere reserve, the Western Ghats, India. J Theo Exp Biol 4: 115-119.
- Harborne JB (1973) Phytochemical methods: a guide to modern techniques of plant analysis. Chapman & Hall, New York.
- Sahu R, Saxena J (2013) Screening of total phenolic and flavonoid content in conventional and non-conventional species of Curcuma. J Pharm Phytochem 2: 176-179.
- Osuagwu GG, Onwuegbuchulam NP (2015) The phytochemical screening and antimicrobial activity of the leaves of Monodora myristica, (Gaertn) Dunal, Acanthus montanus (Ness) T. anders and Alstoniabonnei De Wild. Int J Pharm Phytochem Res 2: 85-102.
- Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40: 945-948.
- Tarsisius B, Robert B, Muhammad N (2013) Tests confirm suitability of Ugandan soils for commercial growing of Artemisia annua Linn. African J Agric Res 8: 4565-4572.
- Kumari OS, Rao NB (2015) Phytochemical analysis of Artemisia nilagarica leaf extract. European J Pharm Med Res 2: 366-370.
- Rani NP, Moorthi C, Senthamarai R, Kandasamy K (2012) A study to explore the pharmacognostic and phytochemical screening of Artemisia nilagarica leaves found in Nilgiri district of Tamil Nadu. Int J Pharm Pharm Sci 4: 441-447.
- Parameswari P, Devika R (2014) Phytochemical screening of bioactive compounds of Artemisia nilagarica (Clarke) Pamp. J Chem Pharm Sci 7: 351-353.
- Rashmi TR, Francis MS, Murali S (2014) Determination of artemisinin in selected Artemisia L. species by HPLC. Indo Am J Pharm Res 4: 2637-2644.