Journal of Oral Biology
Download PDF
Case Report
*Address for Correspondence: Sang Choon Cho, Clinical Assistant Professor and Director of Advanced Program for International Dentist in Implant Dentistry and Co-Director of Clinical research, Ashman Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, USA, E-mail: scc2@nyu.edu
Citation: Dagba SA, Alodadi A, Suzuki T, Sadda R, Chan KC, et al. Implant Placement in a Patient Presenting Avascular Bone Necrosis: A Histological Report. J Oral Bio. 2016; 3(1): 6.
Copyright © 2016 Dagba SA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 3, Issue: 1
Submission: 09 Feb, 2016 | Accepted: 02 May, 2016 | Published: 09 May, 2016
The patient presented with a history of liver transplant, after being diagnosed with biliary cirrhosis 8 months earlier. Therefore, she was on immunosuppressant, Tacrolimus 3 mg (Prograf, Astellas Pharma, US). She was also taking hydroxychloroquine sulfate (Plaquenil, Sanofi -Aventis, US) for systemic lupus erythematosus since 1997, as well as Rivaroxaban (Xarelto, Janssen Pharmaceuticals) for deep vein thrombosis since 2000. The patient also presented a history of bisphosphonate medication for 3 months in 2011 (Fosamax, Merck). She never had undergone radiotherapy of the jaws.
The stage 2 was done end of March 2014, and 2 healing abutments 3.5 x 5 mm were placed (Figure 16). No abnormality of the area was noted, the gingiva in the anterior mandible didn’t present any sign of infl ammation or infection. The patient was then ready to load the implants. Three weeks later, two Locator® attachments (Zestanchors) were placed. The patient was comfortable and did not report any pain or inconvenience. It has been confirm by another follow-up, 2 weeks after, in which a proper soft tissue healing was noticed.
At no point during the healing process an exposure of the implants was observed, the surrounding soft tissues were healthy and neither inflammation nor infection was noticed. The implants survival in that case can be attributed to the fact that they were placed enough away from the AVN. Especially the left implant, which was placed in #22 site after a communication between the #23 site and the AVN area was find out that avoids any compromising of the blood supply, even after the exposure of the AVN, and allowed preserving the bone around the implants. Another reason explaining the implants survival is to have approach the case with a 2-stage procedure, although suffi cient primary stability of the implants was obtained (Figures 25-27) [14].
Considering that case, the worst decision would probably have been to close the flap without placing the implants. We would have to wait for the site healing and the patient would have been exposed to another surgery [15]. The best choice would certainly have been to remove the AVN bone at the moment of the implant surgery. It wasn’t done because that was an unexpected fi nding. When the AVN was found, its dimension wasn’t known. It wasn’t possible then to know how deep the AVN was (Figure 2). Without having more information concerning that bone, the risk of weakening or fracture of the jaw was considered, and the decision of closing the site without removing the bone was preferred [16].
Implant Placement in a Patient Presenting Avascular Bone Necrosis: A Histological Report
Alex Steeve Dagba1, Abdullah Alodadi1, Takanori Suzuki2, Raid Sadda3, King Chong Chan4 and Sang Choon Cho5
- 1Ashman Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, New York, USA
- 2Ashman Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, USA
- 3Department of Oral and Maxillofacial Surgery, New York University College of Dentistry, New York, USA
- 4Department of Oral and Maxillofacial Pathology, Radiology and Medicine, New York University College of Dentistry, New York, USA
- 5Ashman Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, USA
*Address for Correspondence: Sang Choon Cho, Clinical Assistant Professor and Director of Advanced Program for International Dentist in Implant Dentistry and Co-Director of Clinical research, Ashman Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, USA, E-mail: scc2@nyu.edu
Citation: Dagba SA, Alodadi A, Suzuki T, Sadda R, Chan KC, et al. Implant Placement in a Patient Presenting Avascular Bone Necrosis: A Histological Report. J Oral Bio. 2016; 3(1): 6.
Copyright © 2016 Dagba SA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 3, Issue: 1
Submission: 09 Feb, 2016 | Accepted: 02 May, 2016 | Published: 09 May, 2016
Abstract
Patients who are exposed to immunosuppressant medication and antiresorptive therapy such as bisphosphonates present an important risk for developing osteonecrosis, notably in the jaws. Osteonecrosis is a complex disease still not well understood which involve different cell-types. This case reports an implant placement in a 69-year old female who presented an avascular necrotic bone localized in the anterior mandible. The patient had a medical history of liver transplant and osteoporosis. A 14-month follow-up, after implants placement and removal of the necrotic bone, showed a satisfactory healed ridge and soft tissue healing associates with a survival of the implants. In conclusion, it seems that implant surgery is not necessarily contraindicated for patients who presented avascular necrosis of bone.Keywords
Avascular necrotic bone; Osteonecrosis; Implant; Mandible; Liver Transplantation; Bisphosphonates; Bone remodelingIntroduction
Avascular necrosis (AVN), also described as osteonecrosis, ischemic necrosis, or either non-viable dense sclerotic bone, is a complex disease still not well understood. It is a pathology associated with different conditions such as trauma, drugs, cigarette smoking, alcohol consumption, metabolic disorder, organ transplantation or connective tissue disease, and which involves multiple tissues and cell-types.Several etiologies can be considered including bisphosphonate (BP) toxicity to oral epithelium, altered wound healing after tooth extraction or suppression of angiogenesis and bone turnover. Patients exposed to BP have a higher risk to develop avascular necrosis of the bone. In the literature it is one of the mostly reported side effects for BP. The main hypothesis to explain this phenomenon is the important inhibition for BP on the osteoclastic activity, which by extension can cause an inhibition of the normal bone turnover surgical procedures such as extractions.
Since the first publication in 2003, few cases concerning a nonexposed avascular bone necrosis have been described in the literature. Up to the authors’ knowledge, no cases reporting implant placement with an avascular necrotic bone finding have been published. The purpose of this case report is to describe a case of an avascular bone finding on a liver transplant patient who was treated by bisphosphonate orally, and who presented avascular necrosis in themandibular bone.
Report of a Case
A 69 year-old afro-american female patient, treated with two complete dentures, presented to the NYUCD Implant department in order to improve the comfort of her mandibular denture. Decision has been made to increase the retention of the mandibular denture by placement of two implants in the anterior mandible area and to deliver therefore a mandibular overdenture (Figure 1).The patient presented with a history of liver transplant, after being diagnosed with biliary cirrhosis 8 months earlier. Therefore, she was on immunosuppressant, Tacrolimus 3 mg (Prograf, Astellas Pharma, US). She was also taking hydroxychloroquine sulfate (Plaquenil, Sanofi -Aventis, US) for systemic lupus erythematosus since 1997, as well as Rivaroxaban (Xarelto, Janssen Pharmaceuticals) for deep vein thrombosis since 2000. The patient also presented a history of bisphosphonate medication for 3 months in 2011 (Fosamax, Merck). She never had undergone radiotherapy of the jaws.
At the intraoral examination prior to the implant placement procedure, there was neither sign of infection, no swelling, nor bone exposure. The panoramic radiograph taken pre-operatively didn’t show any abnormal finding either. In November 2013, two Nobel Biocare Narrow Platform implants (3.5 x 10 mm) were placed in the mandible, following the manufacturer recommendation. The patient was prescribed amoxicillin 500 mg tid for 7 days to be started the morning of surgery. Upon opening the flap, what seemed to be avascular bone (white bone) was noticed in the anterior mandible, that avascular bone was outside of the planned implant site (Figure 2). No knowing how far it spread throughout the mandible, the decision to not remove it was made. Concerning the implants, they were placed away from that area. However, aft er the initial drilling on the left side, a communication with the avascular bone was noticed, so the implant was placed further distally, between the canine and the first premolar area, with a sufficient primary stability (25 Ncm) (Figure 3). Following cover screws placement, sutures were accomplished using 4-0 Vicryl (Figure 4). A day 4 post-operative follow-up showed that the healing process of the gingiva above the implants had started, and there was an adequate soft tissue primary closure. However, the AVN area was not healed and characterized by a reopening of the surgical site (Figure 5). Decision has been made to re-suture after de-epithelization the soft tissue surrounding that area (Figure 6). 7 days later, the follow-up still showed no evidence of healing in that area (Figures 7 and 8). A computed tomography (CT) was taken and it was reported that “the areas of bone change consist of well defined; sclerotic; calcified masses with radiolucent rim appear to have sequestered. The cancellous bone of the anterior mandible is sclerotic”. It was then decided to remove that area by sequestrectomy and surgical debridement under local anesthesia and antibiotic therapy consisting of amoxicillin 500 mg tid for 7 days. During that procedure the zones surrounding the implants were not involved (Figures 9-12). The sequestrum was sent to histologic analysis, which confirmed “an AVN consistent with sequestrum” (Figures 13 and 14). The follow-up appointments revealed a primary closure associated with a good soft tissue healing, and no pain or discomforts were reported from the patient (Figure 15).
Discussion
Risk AssessmentThe half-life of BP in bone is 11 years, and the prevalence of Osteonecrosis of the jaw (ONJ) stage 0 is may be under estimated [1]. Indeed 30% of the ONJ are non-exposed and asymptomatic. Plus, to date ONJ stage-0 has been reported only on patient BP treated [2].
The major action of bisphosphonates is creating disruption and apoptosis of mature osteoclasts and also osteoclast precursors in the bone marrow [3]. BP leads to hyper-mineralized lamina dura formation by using unremodeled bone that reduces the resorption phase of lamina dura remodelling. Sometimes, when the bisphosphonate dose accumulated in the lamina dura reach a certain level, it can result in a necrotic bone and later on in an exposure. This explains why bone exposure of bisphosphonate-induced osteonecrosis always begins in the alveolar bone or over the remodelling surface of a torus. Bisphosphonate treated patient must be considered as patient who present a potential risk of AVN [4].
Moreover, the patient is an organ (liver) transplant also increases the risk of osteonecrosis. It appears that the immunosuppressive treatment can induce dysfunction of normal bone over time and eventually osteonecrosis. Thus a steroid-induced hypercoagulation state can compromise the blood supply and generate an ischemic bone necrosis [5].
To differentiate between Bisphosphonate Related Osteonecrosis of the Jaw (BRONJ) from other necrotic bone diseases, the American Association of Oral and Maxillofacial Surgery (AAOMS) has introduced three requirements for the clinical diagnosis of BRONJ:
1) Exposed necrotic bone of the jaw for at least 8 weeks.
2) Patient with a medical history of bisphosphonate therapy.
3) No evidence of radiation therapy of the head and neck region [6].
Management of the case
Patients treated with oral bisphosphonates have less chance to develop AVN than those treated with IV bisphosphonates. The risk of AVN, in patients taking oral bisphosphonates, may be associated with prolonged therapy for more than 3 years [7]. Currently, there are no eff ective treatments for AVN. A variety of treatment modalities have been reported in the literature, including conservative therapy, various types of surgery techniques, hyperbaric oxygen, ozone and laser therapy. The current management guidelines are often time based on expert opinions, because of the lack of longitudinal prospective and randomized clinical trials [8].
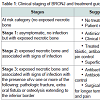
Several classification systems have been proposed to categorize BRONJ. However, AAOMS staging system is the most widely used. It differentiates between four stages of BRONJ, with specific therapeutic recommendations for each BRONJ stage (Table 1) [9].
The effectiveness of hyperbaric oxygen therapy is very controversial. Some reports [10], did not recommend using hyperbaric oxygen, other reports [11], instead, have found it useful. Laser and Ozone therapy have been recommended based on their antimicrobial and biostimulation prosperities [12].
Considering the imaging, in high risk of ONJ cases it seems primordial to have precise information. Panoramic radiograph can tend to under defi ned AVN, especially in the anterior mandible sector (Figures 17 and 18). CBCT is recommended to analyze the bone anatomy in detail, and planning any surgical procedure in the best way. During implant surgery, peri-apical radiographies are also recommended to approach and analyze the relationship between the implants and the osteonecrosis (Figures 19-24) [13].
Later on, at the time of the bone removal a full thickness flap extended behind the #27 implants site was raised. The main purpose of the latter was to assess the AVN, but it also allowed following up and controlling that no bone loss was noticed around the implant, because that implant was more close to the AVN. The AVN was carefully removed and insuring any damage in the implants areas [17]. Thus it seems that as long as the implants are placed away from the AVN area and are covered, the success rate of the implants placement procedure is not affected.
References
- Aghaloo TL, Dry SM, Mallya S, Tetradis S (2014) Stage 0 osteonecrosis of the jaw in a Patient on denosumab. J Oral Maxillofac Surg 72: 702-716.
- Junquera L, Gallego L (2008) Nonexposed bisphosphonate-related osteonecrosis of the jaws: another clinical variant? J Oral Maxillofac Surg 66: 1516-1517.
- Pichardo SE, Kuypers SC, van Merkesteyn JP (2013) Denosumab osteonecrosis of the mandible: a new entity? A case report. J Craniomaxillofac Surg 41.
- Kuijpers SC, de Jong E, Hamdy NA, van Merkesteyn JP (2011) Initial results of the treatment of diffuse sclerosing osteomyelitis of the mandible with bisphosphonates. J Craniomaxillofac Surg 39: 65-68.
- Trivellato AE, Ribeiro MC, Sverzut CE, Bonucci E, Nanci A, et al. (2009) Osteopetrosis complicated by osteomyelitis of the maxilla and mandible: light and electron microscopic findings. Head Neck Pathol 3: 320-326.
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, et al. (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg 67(5 Suppl): 2-12.
- Ficarra G, Beninati F (2007) Bisphosphonate-related osteonecrosis of the jaws: an update on clinical, pathological and management aspects. Head Neck Pathol 1: 132-140.
- Marx RE, Sawatari Y, Fortin M, Broumand V (2005) Bisphosphonateinduced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63: 1567-1575.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons (2007) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 65: 369-376.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62: 527-534.
- Freiberger JJ, Padilla-Burgos R, Chhoeu AH, Kraft KH, Boneta O, et al. (2007) Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: a case series. J Oral Maxillofac Surg 65: 1321-1327.
- Agrillo A, Petrucci MT, Tedaldi M, Mustazza MC, Marino SM, et al. (2006) New therapeutic protocol in the treatment of avascular necrosis of the jaws. J Craniofac Surg 17: 1080-1083.
- Alsufyani NA, Lam EW (2011) Osseous (Cemento-osseous) dysplasia of the jaws: clinical and radiographic analysis. J Can Dent Assoc 77: b70.
- An SY, Shin HI, Choi KS, Park JW, Kim YG, et al. (2013) Unusual osteoid osteoma of the mandible: report of case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 116: e134-140.
- Li H, Zhang J, He JW, Wang K, Wang GS, et al. (2012) Symptomatic osteonecrosis of the femoral head after adult orthotopic liver transplantation. Chin Med J (Engl) 125: 2422-2426.
- Horiuchi H, Hashikura Y, Hisa K, Saito N, Ikegami T, et al. (2004) Osteonecrosis of the femoral head in Japanese adults after liver transplantation: a preliminary report. J Orthop Sci 9: 119-121.
- Marx RE (2014) A Decade of bisphosphonate bone complications: what it has taught us about bone physiology. Int J Oral Maxillofac Implants 29: e247-258.