Journal of Oral Biology
Download PDF
Research Article
*Address for Correspondence: Jeong-Dan Cha, Professor, Institute of Jinan Red Ginseng, 520-9 Banwolri, Jinan-eup, Jinan-gun, Jeollabuk-do, 567-801 South Korea, Tel: +82-63-432-0913; Fax: +82-63-432-0910; E-mail: joungdan@ijrg.re.kr
Citation: Cha SM, Han SB, Lee YS, Cha JD. Synergistic Effect of the Ethanol Extract of Alismatis rhizoma against Oral Pathogens. J Oral Bio. 2015;2(1):7.
Copyright © 2014 Cha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 2, Issue: 1
Submission: 11 February 2015 | Accepted: 06 March 2015 | Published: 10 March 2015
or Soxhlet, are often time-consuming and expensive, with low yield of the polysaccharides of A. rhizoma or even loss of some of their pharmacological activities [33,34].
and S. downei. The ARE with gentamicin (1/2 MIC) combination was bactericidal effect in S. sanguinis, S. downei, S. anginosus, and S. gordonii with all killing up to 12 h exposure.
Synergistic Effect of the Ethanol Extract of Alismatis rhizoma against Oral Pathogens
Su-Mi Cha1, Su-Beom Han1, Young-Soo Lee2 and Jeong Dan Cha3*
- 1Department of Oral Microbiology and Institute of Oral Bioscience, Chonbuk National University, Jeonju, South Korea
- 2Department of Dental Hygiene, Sun Moon University, Republic of Korea
- 3Department of Efficacy Research, Institute of Jinan Red Ginseng, South Korea
*Address for Correspondence: Jeong-Dan Cha, Professor, Institute of Jinan Red Ginseng, 520-9 Banwolri, Jinan-eup, Jinan-gun, Jeollabuk-do, 567-801 South Korea, Tel: +82-63-432-0913; Fax: +82-63-432-0910; E-mail: joungdan@ijrg.re.kr
Citation: Cha SM, Han SB, Lee YS, Cha JD. Synergistic Effect of the Ethanol Extract of Alismatis rhizoma against Oral Pathogens. J Oral Bio. 2015;2(1):7.
Copyright © 2014 Cha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Oral Biology | ISSN: 2377-987X | Volume: 2, Issue: 1
Submission: 11 February 2015 | Accepted: 06 March 2015 | Published: 10 March 2015
Abstract
Background: Alismatis rhizoma or Alisma orientale (Zexie in Chinese), the dried rhizome of Alisma orientale Juzepzuk (Alismataceae), is known to have diuretic and damp-heat clearing actions and has been used for the treatment of dysuria, edema, urinary tract infections, the retention of fluid and phlegm, and vertigo in Asia and Europe.Methods: In this study, the combination effect of the ethanol extract of Alismatis rhizoma (ARE) was evaluated against oral bacteria, either alone or with antibiotics, via broth dilution method and checkerboard and time kill assay.
Results: In these results, MIC/MBC values for ARE against all the tested bacteria ranged between 313-5000/625-5000 microg/mL, for ampicillin 0.25-32/0.5-64 microg/mL and for gentamicin 4-32/8-64 microg/mL respectively. Furthermore, the MIC and MBC were reduced to one half-twelve as a result of the combination of ARE with antibiotics. 1-2 hours of treatment with 1/2 MIC of ARE with 1/2 MIC of antibiotics resulted from an increase of the rate of killing in units of CFU/ mL to a greater degree than was observed with alone.
Conclusions: These results suggest that the ARE is important in the antibacterial actions of oral pathogen agents.
Keywords
Alismatis rhizome; Antibacterial activity; Oral pathogen bacteria; Synergistic effect; Minimum inhibitory concentrations (MICs); Minimum bactericidal concentrations (MBCs)Abbreviations
ARE: The Ethanol Extract of Alismatis rhizome; MICs: Minimum Inhibitory Concentrations; MBCs: Minimum Bactericidal Concentrations; CFU: Colony Forming Unit; FIC index: Fractional Inhibitory Concentration index; FBC index: Fractional Bactericidal Concentration indexIntroduction
Oral diseases are major health problems with dental caries and periodontal diseases among the most important preventable global infectious diseases [1,2]. Oral health influences the general quality of life and poor oral health is linked to chronic conditions and systemic diseases [3]. The association between oral diseases and the oral microbiota is well established [4]. The development of dental caries involves acidogenic and acid uric gram-positive bacteria, primarily the mutans streptococci (Streptococcus mutans and S. sobrinus), lactobacilli, and actinomycetes, which metabolize sucrose to organic acids (mainly lactic acid) that dissolve the calcium phosphate in teeth, causing decalcification and eventual decay [5,6]. In contrast, periodontal diseases are sub gingival conditions that have been linked to anaerobic gram-negative bacteria such as Porphyromonas gingivalis, Actinobacillus sp, Prevotella sp, and Fusobacterium sp. [7,8]. Antibiotics such as ampicillin, chlorhexidine, erythromycin, penicillin, tetracycline, and vanco- mycin have been very effective in preventing dental caries [9]. Of the selected putative periodontal species, strains of Prevotella intermedia, Fusobacterium nucleatum and to the first time, Tannerella forsythia, were β-lactamase positive, with P. intermedia being the most frequently detected enzyme positive species [10]. Sub gingival isolates of P. gingivalis,P. intermedia and F. nucleatum in a group of subjects increase in the MIC values of tetracycline [11]. This clinical observation led to studies that established metronidazole as an important antibiotic for anaerobic infection. Since then, this compound has also played an important role in treating anaerobe related infection in the oral cavity, abdomen, and female genital tract, among others [12]. Oral bacteria have been reported to show increased resistance towards common antibiotics such as penicillin, cephalosporin, erythromycin, tetracycline, and metronidazole which have been used therapeutically for the treatment of oral infection [13,14]. The increase in resistance and adverse effects has lead researchers to explore novel anti-infective herbal compounds which could be used for effective treatment of oral diseases [15,16].Natural medicine is a precious resource of therapeutically active compounds and has increasingly attracted the attention of researchers [17]. Alismatis rhizoma or Alisma orientale (Zexie in Chinese), the dried rhizome of Alisma orientale Juzepzuk (Alismataceae), is known to have diuretic and damp-heat clearing actions and has been used for the treatment of dysuria, edema, urinary tract infections, the retention of fluid and phlegm, and vertigo in Asia and Europe [18]. Phytochemistry studies have shown that protostane triterpenes, guaiane sesquiterpenes, alisol B, polysaccharides, and kaurane diterpenes are the major chemical components [19,20], its components have been imported antbioactivities, including anti-hepatitis, antiinflammatory, hypo-glycemic, and immunological effects [20-23]. A new triterpenoid from Alisma orientale exhibits their antibacterial effect against antibiotic resistant strains, Enterococcus faecium, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa [23]. Many plant-derived medicines used in traditional medicinal systems have been recorded in pharmacopeias as agents used to treat infections and a number of these have been recently investigated for their efficacy against oral microbial pathogens [24-26].
In this study, we investigated the synergistic antibacterial activity of the ethanol extact of Alismatis rhizoma (ARE) in combination with existing antimicrobial agents against oral bacteria.
Materials and Methods
Plant material and preparation of crude plant extractAlismatis rhizoma was purchased from the herbal medicine cooperative association of Jeonbuk Province, Korea, in March 2005. The identity was confirmed by Dr. Bong-Seop Kil, College of Natural Science, and Wonkwang University. Voucher specimens (JD-JRG1) were deposited in the Herbarium of Institute of Jinan Red Ginseng. The dried and powered of A. rhizoma were extracted by refluxing the samples with ethanol (EtOH) for 4 h at 80 °C three times. The solvent extracts were removed under vacuum on a rotary evaporator at 40 °C. The extracts were then dissolved in 10% dimethyl sulfoxide (DMSO) for testing. All the extracts were kept at 4 °C in the dark until further use.
Bacterial strains
The oral bacterial strains used in this study were: cariogenic bacterial strains, Streptococcus mutans ATCC 25175 (American Type Culture Collection), Streptococcus sanguinis ATCC 10556, Streptococcus. sobrinus ATCC 27607, Streptococcus ratti KCTC (Korean Collection for Type Cultures) 3294, Streptococcus criceti KCTC 3292, Streptococcus parasanguinis KCOM 1497 (Korean Collection for Oral Microbiology), Streptococcus downei KCOM 1165, Streptococcus anginosus ATCC 31412, and Streptococcus gordonii ATCC 10558 and periodontopathogenic bacterial strains, Actinobacillus actinomycetemcomitans ATCC 43717, Fusobacterium nucleatum ATCC 10953, and Porphylomonas gingivalis ATCC 33277. Brain-Heart Infusion (BHI) broth supplemented with 1% yeast extract (Difco Laboratories, Detroit, MI) was used for cariogenic bacterial strains (facultative anaerobic bacteria). For periodontopathogenic bacterial strains (microaerophilic and obligate anaerobic bacteria), BHI broth containing hemin 1 μg/ml (Sigma, St. Louis, MO, USA) and menadione 1 μg/ml (Sigma) was used.
Minimum inhibitory concentrations/minimum bactericidal concentrations assay
The minimum inhibitory concentrations (MICs) were determined for ARE by the broth dilution method [27], and were carried out in triplicate. The antibacterial activities were examined after incubation at 37 °C for 18 h (facultative anaerobic bacteria), for 24 h (microaerophilic bacteria), and for 1-2 days (obligate anaerobic bacteria) under anaerobic conditions. MICs were determined as the lowest concentration of test samples that resulted in a complete inhibition of visible growth in the broth. MIC50s and MIC90s, defined as MICs at which, 50 and 90%, respectively of oral bacteria were inhibited, were determined. Following anaerobic incubation of MICs plates, the minimum bactericidal concentrations (MBCs) weredetermined on the basis of the lowest concentration of acacetin that kills 99.9% of the test bacteria by plating out onto each appropriate agar plate. Ampicillin (Sigma) and gentamicin (Sigma) were used as standard antibiotics in order to compare the sensitivity of ARE against oral bacteria.
Checker-board dilution test
The antibacterial effects of a combination of ARE, which exhibited the highest antimicrobial activity, and antibiotics were assessed by the checkerboard test as previously described [27,28]. The antimicrobial combinations assayed included ARE with ampicillin or gentamicin. Serial dilutions of two different antimicrobial agents were mixed in cation-supplemented Mueller-Hinton broth, which is the medium usually used for dilution antimicrobial susceptibility tests. This medium is supplemented with calcium and magnesium salts to produce correct MICs with aminoglycosides and Pseudomonas aeruginosa [29]. After 24-48 h of incubation at 37 °C, the MICs were determined to be the minimal concentration at which there was no visible growth and MBCs were determined on the basis of the lowest concentration of ARE that kills 99.9% of the test bacteria by plating out onto each appropriate agar plate. The fractional inhibitory concentration (FIC)/ fractional bactericidal concentration (FBC) index was calculated according to the equation: FIC/FBC index=FIC/ FBCA+FIC/FBCB=(MIC/MBC of drug A in combination/MIC/MBC of drug A alone)+(MIC/MBC of drug B in combination/MIC/MBC of drug B alone). The FIC and FBC index are the sum of the FICs and FBCs of each of the drugs, which in turn is defined as the MIC and MBC of each drug when it is used in combination divided by the MIC and MBC of the drug when it is used alone. The interaction was defined as synergistic if the FIC and FBC index was less than or equal to 0.5, additive if the FIC and FBC index was greater than 0.5 and less than or equal 1.0, indifferent if the FIC and FBC index was greater than 1.0 and less than or equal to 2.0, and antagonistic if the FIC and FBC index was greater than 2.0.
Time-kill curves
Bactericidal activities of the drugs under study were also evaluated using time-kill curves on oral bacteria. Tubes containing Mueller-Hinton supplemented to which antibiotics had been added at concentrations of the 1/2 MIC were inoculated with a suspension of the test strain, giving a final bacterial count between 6~7×106 CFU/mL. The tubes were thereafter incubated at 37 °C in an anaerobic chamber and viable counts were performed at 0, 0.5, 1, 2, 3, 6, 9, 12,18 and 24 h after addition of antimicrobial agents, on agar plates incubated for up to 48 h in anaerobic chamber at 37 °C. Antibiotic carryover was minimized by washings by centrifugation and serial 10-fold dilution in sterile phosphate-buffered saline, pH 7.3. Colony counts were performed in duplicate, and means were taken. The solid media used for colony counts were BHI agar for streptococci and BHI agar containing hemin and menadione for P. gingivalis.
Results and Discussion
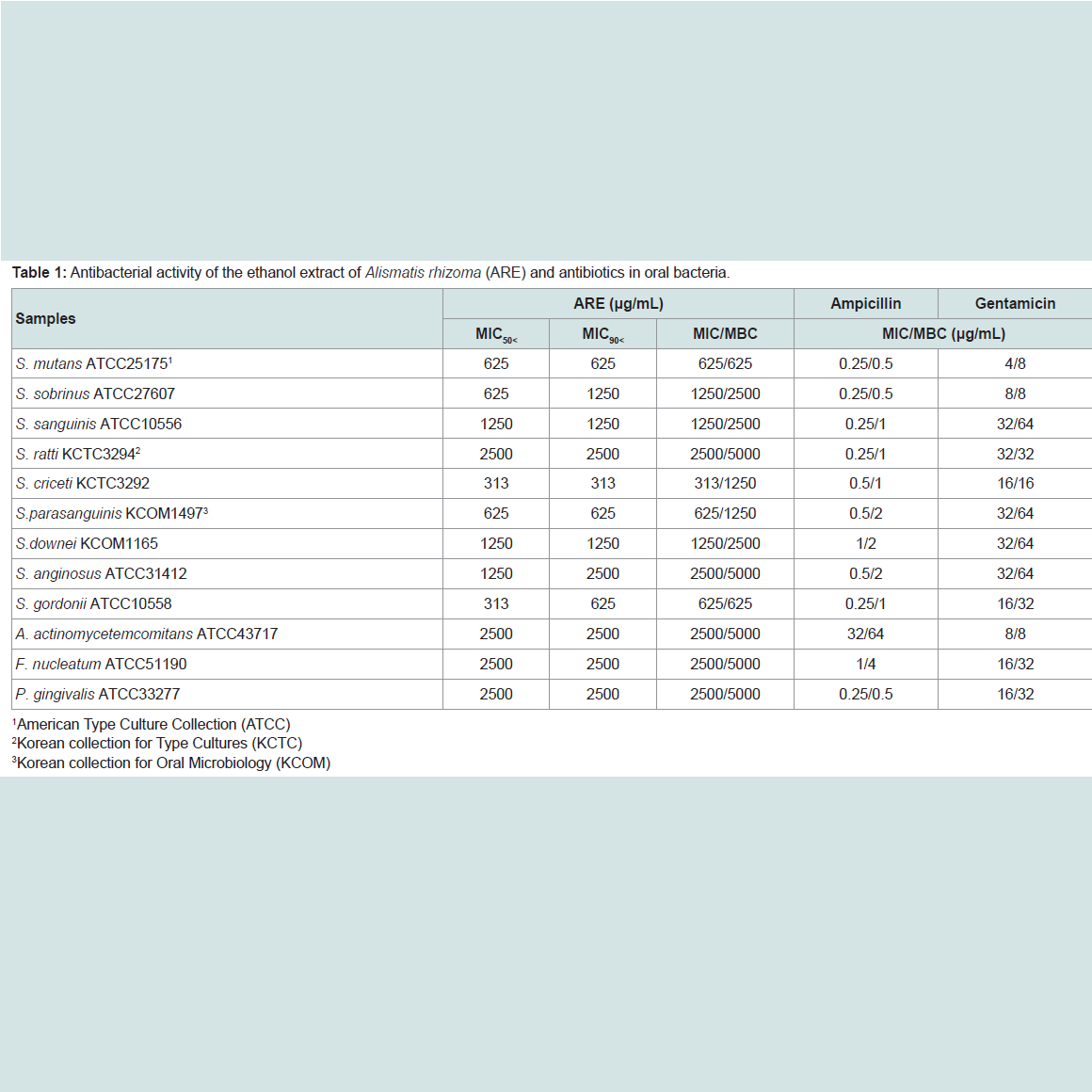
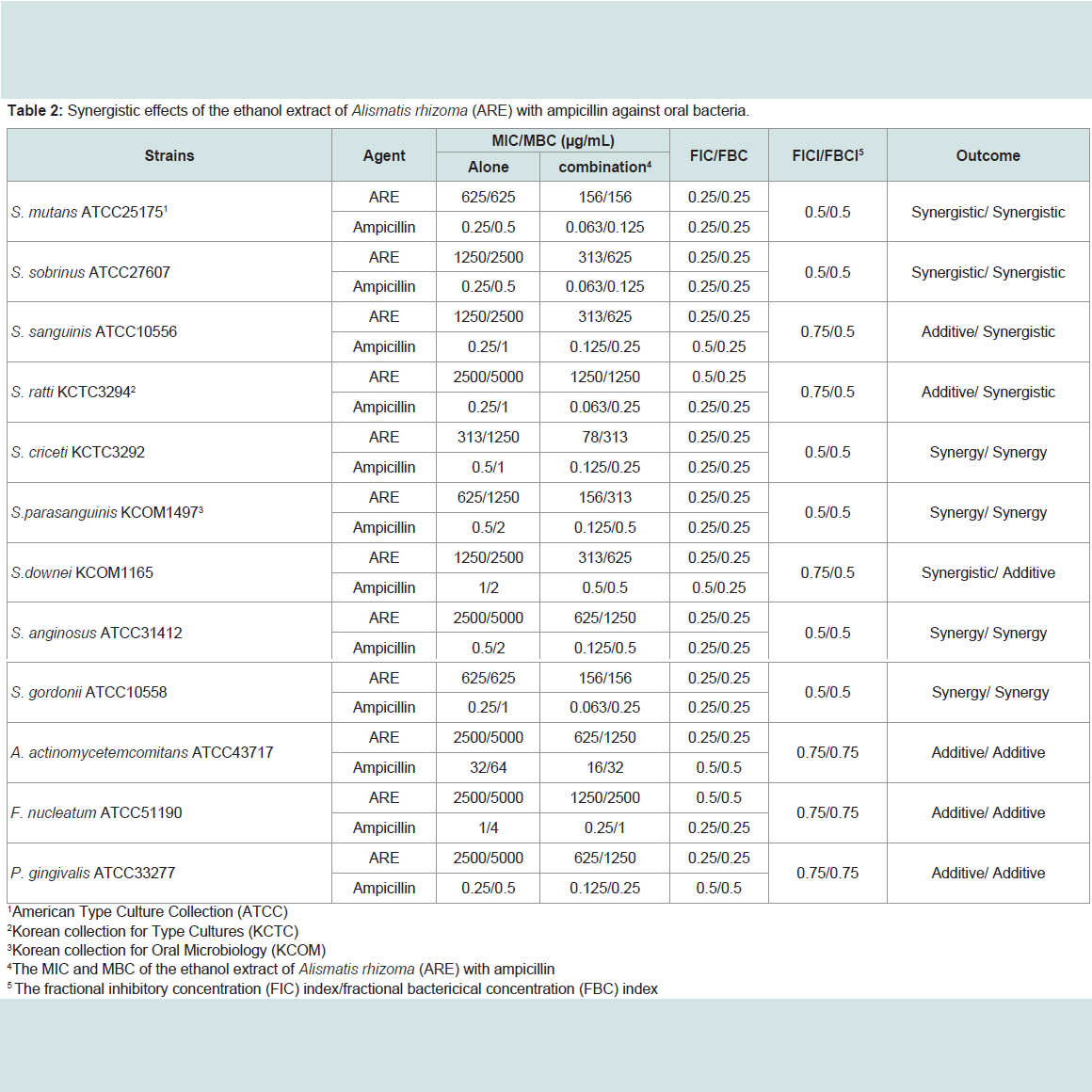
The ARE was evaluated for their antimicrobial activities against twelve common bacterial species present in the oral cavity. The results of the antimicrobial activity showed that the ARE exhibited antimicrobial activities against cariogenic bacteria (MICs, 313 to 2500μg/mL; MBCs, 625 to 5000 μg/mL), against periodonto pathogenic bacteria (MICs, 2500 μg/mL; MBCs, 5000 μg/mL) and for ampicillin, either 0.25/0.5 or 32/64 μg/mL; for gentamicin, either 4/8 or 32/64μg/mL on tested all bacteria (Table 1). The range of MIC50 and MIC90 of the ARE were from 313 to 2500 μg/mL and 313 to 2500 μg/mL, respectively. The ARE showed stronger antimicrobial activity against S. mutans, S. creceti, S. parasanguinis, and S. gordonii than another bacteria and the range of MIC50 and MIC90 were 313-625 μg/mL and 313-625 μg/mL.Natural products are a major source of chemical diversity and have provided important therapeutic agents for many bacterial diseases [27,28,30,31]. Combinations of some herbal materials and different antibiotics might affect the inhibitory effect of these antibiotics [30,31]. The synergistic effects of the ARE alone or with antibiotics were evaluted in oral bacteria (Table 2 and Table 3). In combination with the ARE, the MIC for ampicillin was reduced ≥4-fold in cariogenic bacteria, S. mutans, S. sobrinus, S. criceti, S. parasanguinis, S. anginosus, and S. gordonii, producing a synergistic effect as defined by FICI ≤ 0.5. The MBC for ampicillin was shown synergistic effects in all cariogenic bacteria by FBCI ≤ 0.5, but not the periodontopathogenic bacteria by FBCI ≥ 0.75 (additive) (Table 2). In combination with the ARE, the MIC for gentamicin was reduced ≥4-16-fold in all tested bacteria expect S. sanguinis, S. ratti, S. criceti, and S. gordonii by FICI ≥ 0.75 and MBC in S. ratti, S.criceti, and S. gordonii by FBCI ≥ 0.75 (Table 3).
Recently, interest in A. rhizoma is growing, because many of its components, such as triterpene, alisol B, and especially polysaccharides, have been reported to have important bioactivities, including anti-hepatitis, anti-inflammatory, hypoglycemic and immunological effects [20-22]. Four triterpenes (alisol A, alisol A monoacetate, alisol B, and alisol B monoacetate) isolated from the A. rhizoma have show anti-complementary and antibacterial activity against antibiotic resistant strains [20,32]. The polysaccharides of A. rhizoma have been documented for their effects of against lipid peroxidation, which are potentially important for human health [33]. Conventional methods, such as those by hot water, immersion
Figure 1: Time-kill curves of MIC of ARE alone and its combination with 1/2 MIC of Amp or Gen against. S. mutans, S. sanguinis, S. sobrinus, S. ratti, S. criceti, and S. parasanguinis. Bacteria were incubated with ARE (●), ARE + Amp (○), and ARE + Gen (▼) over time. Data points are the mean values±S.E.M. of six experiments. CFU, colony-forming units.
The bacterial effect of the ARE with ampicillin or gentamicin against oral bacteria was confirmed by time-kill curve experiments.The ARE (MIC or 1/2 MIC) alone resulted rate of killing increasing or not changing in CFU/ml at time-dependent manner, with a more rapid rate of killing by ARE (1/2 MIC) with ampicillin (1/2 MIC) or gentamicin (1/2 MIC) (Figure 1 and Figure 2). The ARE with ampicillin (1/2 MIC) combination was bactericidal effect up to and beyond 12 h exposure with all killing compared to ARE alone in S. sobrinus
Figure 2: Time-kill curves of MIC of ARE alone and its combination with 1/2 MIC of Amp or Gen against S. downei, S. anginosus, S. gordonii, A. actinomycetemcomitans, F. nucleatum, and P. gingivalis. Bacteria were incubated with ARE (●), ARE + Amp (○), and ARE + Gen (▼) over time. Data points are the mean values±S.E.M. of six experiments. CFU, colony-forming units.
Conclusion
In conclusion, these findings suggest that a strong bactericidal effect of ARE was exerted in drug combinations and fulfills the conditions required of a novel cariogenic bacteria and periodontal pathogens, particularly bacteroides species drug and may be useful in the future in the treatment of oral bacteria.References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43: 5721-5732.
- Grossner-Schreiber B, Fetter T, Hedderich J, Kocher T, Schreiber S, et al. (2006) Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: a case-control study. J Clin Periodontol 33: 478-484.
- Chi AC, Neville BW, Krayer JW, Gonsalves WC (2010) Oral manifestations of systemic disease. Am Fam Physician 82: 1381-1318.
- Collins LM, Dawes C (1987) The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res 66: 1300-1302.
- Marsh PD (2006) Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC Oral Health 6: S14.
- Kleinberg I (2002) A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 13: 108-125.
- Lagdive SS, Lagdive SB, Mani A, Anarthe R, Pendyala G, et al. (2013) Correlation of mast cells in periodontal disease. J Indian Soc Periodontol 17: 63-67.
- Ardila CM, López MA, Guzmán IC (2011) Positive correlations between presence of gram negative enteric rods and Porphyromonas gingivalis in subgingival plaque. Acta Odontol Latinoam 24: 15-19.
- Levi ME, Eusterman VD (2011) Oral infections and antibiotic therapy. Otolaryngol Clin North Am 44: 57-78.
- van Winkelhoff AJ1, Winkel EG, Barendregt D, Dellemijn-Kippuw N, Stijne A, et al. (1997) beta-Lactamase producing bacteria in adult periodontitis. J Clin Periodontol 24: 538-543.
- Abu Fanas SH, Drucker DB, Hull PS (1991) Amoxicillin with clavulanic acid and tetracycline in periodontal therapy. J Dent 19: 97-99.
- Falagas ME, Gorbach SL (1995) Clindamycin and metronidazole. Med Clin North Am 79: 845-867.
- Rams TE, Degener JE, van Winkelhoff AJ (2012) Prevalence of β-lactamase-producing bacteria in human periodontitis. J Periodontal Res 48: 493-499.
- Bedenić B, Budimir A, Gverić A, Plecko V, Vranes J, et al. (2012) Compara- tive urinary bactericidal activity of oral antibiotics against gram-positive pathogens. Lijec Vjesn 134: 148-155.
- Feldman M, Santos J, Grenier D (2011) Comparative evaluation of two structurally related flavonoids, iso- liquiritigenin and liquiritigenin, for their oral infection therapeutic potential. J Nat Prod 74: 1862-1867.
- Greenberg M, Dodds M, Tian M (2008) Naturally occurring phenolic antibacterial compounds show effectiveness against oral bacteria by a quantitative structure- activity relationship study. J Agric Food Chem 56: 11151-11156.
- Mishra BB, Tiwari VK (2011) Natural products: an evolving role in future drug discovery. Eur J Med Chem 46: 4769-4807.
- Liu X, Li LS, ZhouY, Song JZ, Zheng YF, et al. (2010) Characterization of protostane triterpenoids in Alisma orientalis by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 24: 1514-1522.
- Lee AY, Park JY, Chun JM, Moon BC, Kang BK, et al. (2013) Optimization of extraction condition for Alisol B and Alisol B acetate in Alismatis rhizoma using response surface methodology. J Liq Chromatogr Relat Technol 36: 513-524.
- Jin HG, Jin Q, Ryun A, Choi H, Lee JH, et al. (2012) A new triterpenoid from Alisma orientale and their antibacterial effect. Arch Pharm Res 35: 1919-1926.
- Dai Y, Hang B, Huang Z, Li P (1991) Anti-inflammatory activities and effect of Alismatis rhizoma on immune system. Zhongguo Zhong Yao Za Zhi 16: 622-625.
- Han CW, Kang ES, Ham SA, Woo HJ, Lee JH, et al. (2012) Antioxidative effects of Alisma orientale extract in palmitate-induced cellular injury. Pharm Biol 50: 1281-1288.
- Jiang ZY, Zhang XM, Zhang FX, Liu N, Zhao F, et al. (2006) A new triterpene and anti-hepatitis B virus active compounds from Alisma orientalis. Planta Med 72: 951-954.
- Choi JS, Ha YM, Joo CU, Cho KK, Kim SJ, et al. (2012) Inhibition of oral pathogens and collagenase activity by seaweed extracts. J Environ Biol 33: 115-121.
- Palombo EA (2011) Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med 2011: 680354.
- Yamamoto H, Ogawa T (2002) Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci Biotechnol Biochem 66: 921-924.
- Cha JD, Jeong MR, Jeong SI, Lee KY (2007) Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens. J Microbiol Biotechnol 17: 858-864.
- Ma XH, Zheng CJ, Han LY, Xie B, Jia J, et al. (2009) Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov Today 14: 579-588.
- Clinical and Laboratory Standards Institute (2006) Approved standard: M7-A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. CLSI, Wayne, Pa.
- Bag A, Bhattacharyya SK, Pal NK, Chattopadhyay RR (2012) In vitro antibacterial potential of Eugenia jambolana seed extracts against multidrug-resistant human bacterial pathogens. Microbiol Res 167: 352-357.
- Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15: 639-652.
- Matsuda H, Tomohiro N, Yoshikawa M, Kubo M (1998) Studies on Alismatis rhizome II. anti-complementary activities of methanol extract and terpene components from Alismatis rhizoma (dried rhizome of Alisma orientale). Biol Pharm Bull 21: 1317-1321.
- Li Q, Qu H (2012). Study on the hypoglycemic activities and metabolism of alcohol extract of Alismatis rhizoma. Fitoterapia 83: 1046-1053.
- Zhao ZY, Zhang Q, Li YF, Dong LL, Liu SL (2015) Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohydr Polym 30: 101-109.