Journal of Microbiology & Microbial Technology
Download PDF
Research Article
*Address for Correspondence: Setareh Mamishi, Department of Pediatric Infectious Disease, Children Medical Center Hospital, School of Medicine, Tehran University of Medical Sciences, No.62, Dr. Gharib’s Street, Keshavarz Boulevard, Tehran, Iran, Tel/Fax: +98 021 6428996; E-mail: smamishi@sina.tums.ac.ir
Citation: Pourakbari B, Mahmoudi S, Sadeghi RH, Movahadi Z, Mamishi S. Evaluation of Epstein - Barr Virus in Kawasaki Patients in an Iranian Referral Hospital. J Microbiol Microb Technol 2016;1(2): 3.
Copyright © 2016 Pourakbari B, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Microbiology & Microbial Technology | Volume: 1, Issue: 2
Submission: 24 May, 2016 | Accepted: 28 June, 2016 | Published: 02 July, 2016
Evaluation of Epstein - Barr Virus in Kawasaki Patients in an Iranian Referral Hospital
Babak Pourakbari1, Shima Mahmoudi1, Reihane Hosseinpour Sadeghi1, Zahra Movahadi2 and Setareh Mamishi1,3*
- 1Pediatrics Infectious Diseases Research Center, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Infectious Disease, School of Medicine, Qom University of Medical Sciences, Qom, Iran
- 3Department of Pediatric Infectious Disease, Children Medical Center Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
*Address for Correspondence: Setareh Mamishi, Department of Pediatric Infectious Disease, Children Medical Center Hospital, School of Medicine, Tehran University of Medical Sciences, No.62, Dr. Gharib’s Street, Keshavarz Boulevard, Tehran, Iran, Tel/Fax: +98 021 6428996; E-mail: smamishi@sina.tums.ac.ir
Citation: Pourakbari B, Mahmoudi S, Sadeghi RH, Movahadi Z, Mamishi S. Evaluation of Epstein - Barr Virus in Kawasaki Patients in an Iranian Referral Hospital. J Microbiol Microb Technol 2016;1(2): 3.
Copyright © 2016 Pourakbari B, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Microbiology & Microbial Technology | Volume: 1, Issue: 2
Submission: 24 May, 2016 | Accepted: 28 June, 2016 | Published: 02 July, 2016
Abstract
Introduction: Kawasaki disease (KD) is an acute febrile systemic vasculitis with unknown etiological agent. It had been suggested that KD might be closely associated with an Epstein-Barr virus (EBV). The present study was carried out with the aim of evaluating the correlation between EBV and KD.Material and methods: This study was carried out on 41 patients that fulfilled the criteria and 50 control individuals in an Iranian referral Children’s Medical Center Hospital.
Results: In our study, 11 KD patients (27%) and 19 control cases (38%) were polymerase chain reaction positive respectively. In addition, 15 KD patients (36.5%) and 16 control cases (32%) were seropositive. These results indicated no significant statistical correlation between KD and EBV.
Conclusion: No association was found between KD and EBV and KD children seem to be infected with EBV similar to normal children of the same age.
Keywords
Kawasaki; EBV; ChildrenIntroduction
Kawasaki disease (KD) is an acute febrile systemic vasculitis affecting children between six months and five years of age with an unknown etiology [1,2]. Although KD is the principal cause of acquired heart disease in children, its etiology still remains unknown [1]. Microbial infection considered as one of the KD causes. However, in spite of much effort, the etiological agent of KD remains unknown [2]. The Epstein-Barr virus (EBV) usually cause asymptomatic disease in young children and consider as primary cause of infectious mononucleosis in some older children and young adults. It has been suggested that KD might be closely associated with an EBV. Association of EBV with KD in previous studies was contradictory. The aim of this study was to determine whether EBV infection is associated with the KD in Iranian children in a referral children medical hospital.Materials and Methods
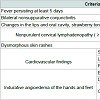
We evaluated the relationship between EBV infection and KD between 2010 and 2011 at Children’s Medical Center, Tehran, Iran.Patient characteristics (sex, age at diagnosis and symptoms) were extracted from patient records. This study was approved by the Ethics Committee of Tehran University of Medical Sciences.Diagnosis of this KD is based on some major symptoms including fever lasting five or more days, conjunctivitis, inflammation of the mucous membranes of the mouth and pharynx, edema, erythema, desquamation of the skin of the hands and feet, a polymorphous rash, and acute nonpurulent cervical lymphadenopathy [3]. For a diagnosisof KD, at least 5 of these 6 clinical criteria or symptoms should be present [4]. Incomplete Kawasaki disease considered in all children with unexplained fever for ≥ 5 days associated with 2 or 3 of the principal clinical features of KD [5].
Forty one cases of KD included in this study and fifty healthy age and sex matched children were used as the control group. In KD patients prior to the start of intravenous immunoglobulin (IVIG)therapy, and from control group a blood sample was taken and blood was then centrifuged for 6 min at 3000 g, the resulting serum immediately frozen in sterile tubes at -80 °C.
We measured antibodies using commercially available kits Viral Capsid Antigen (VCA), EBV ELISA kits (IBL, Hamburg, Germany). For EBV polymerase chain reaction, DNA extracted from the blood samples and nested PCR was performed. Primers appropriate for nested PCR test were chosen from a similar article [6]. The first run of PCR was set in the volume of 50 μl including: 3 μl of extracted DNA, 0.75 μl of MgCl2, 5 μl of 10X PCR buffer, 2U of taq enzyme and 20 pM of each primers for both of virus genotypes (E2P1: AGG GAT GCC TGG ACA CAA GAG, E2P2: TGG TGC TGC TGG TGG YGG CAA T) for the amplification of the 596 bp fragment from virus EBNA-2 gene. The characteristics of thermal cycles was: 94 °C for 3 min, then 40 cycles containing 94 °C for 35 s, 72 °C for 35 s and 72 °C for 45 s. The second run of PCR was carried out by using of PCR products from the first run and by the same condition but with the specialized internal primers Ap1 (TCT TGA TAG GGA TCC GCT AGG ATA), Ap2 (ACC GTG GTT CTG GAC TAT CTG GAT) for A type and Bp1 (CAT GGT AGC CTT AGG ACA TA) and Bp2 (AGA CTT AGT TGA TGC CCT AG) for B type virus.
The PCR product was electrophoresed in 2% agarose gel. The 596 bp product of the common primers, and the 497 bp and 150 bp products of the type-specific primers were visualized under U.V light.
Statistical Analysis
The Statistical Package for the Social Sciences (Windows version 16.0; SPSS Inc, Chicago, US) was used for all analyses. Descriptive statistics were used to summarize patient variables. A p value < 0.05 was taken to be significant.Results
The totals of 41 children who had been diagnosed with KD and were not treated with IVIG during the febrile period were included in the study. The mean age of 41 participants was 53.3 ± 34.6 month (range 2 months to 11 years) with a male-to-female ratio of 1.27 (23 males/18 females). Fifty age-matched healthy children with a meanage of 52.4 ± 32.1 months were used as the control group.The whole patients had fever with the average day of 6.5 and the maximum degree of 40.7 °C. Among 41 patients, 29 cases (71%) and 12 cases (29%) had complete and incomplete KD, respectively. Previous antibiotics usage was observed in 33 patients (80%). Approximately 30% of our KD patients had white blood cell counts > 15000/mm.
After diagnosis, the whole patients were treated with IVIG at a dose of 2 g/kg with aspirin (80 mg/kg) and four patients received the second dose of IVIG.
Clinical features of KD patients are listed in Table 1. The most common symptom of KD in our study was dysmorphous skin rashes (82.9%), changes in the lips and oral cavity, strawberry tongue, diffuse injection of oral and pharyngeal mucosae (80.5%). Elevated erythrocyte sedimentation rate of KD patients was 74 ± 32.1 (range 0 to 150). Out of 41 patients being analyzed, 11 of them (27%) had EBV DNA (type B) in their blood. Frequency of infection by EBV in KD patients by means of PCR and ELISA were 27% (11 patients) and 36.5% (15 patients), respectively. In addition, 32% of control isolates (16 patients) were seropositive for VCA IgG and 38% of them (19 patients) had positive PCR test. The differences between frequency of EBV in cases and controls by both PCR and ELISA methods was not significant (p value = 0.25 via PCR method and p value = 0.64 via ELISA method (Figure 1).
Discussion
Although the etiology of KD remains unknown, there are attractive hypothesis that it is caused by an infectious agent [5]. EBV is one of the most common viruses in human. EBV infections of adolescents and adults usually result in infectious mononucleosiswhile most cases of infants and children with this infection are asymptomatic or have nonspecific symptoms [7]. This disease might spread by intimate contact between susceptible persons and asymptomatic EBV shedders.EBV seropositivity among children has different geographic variation. Low income and crowded family conditions consider as one of the vital factors that increase the likelihood of being EBV seropositive in children [8]. It has been reported that the positive rate among children in Asia is much higher than Western countries [5]. Poor socioeconomic conditions have been associated with early primary EBV infection, whereas late primary EBV infection is seen inpopulations of high socioeconomic status [8]. The majority of primary EBV infections are self-limited clinical syndrome and asymptomatic.
Despite the relationship between KD and EBV have been studied in many cases, studies are not uniform in their conclusions [9-18]. Because the prevalence of EBV is closely related to age, we carefully selected the same-aged controls. The results of this study indicate that nearly one third of our population (both patients and control) not only were seropositive for EBV but also had positive polymerase chain reaction for EBV genome.
Several studies in Japan reported sharp contrast between EBV seropositivity of KD patients and control groups [9,12]. In Huang Y et al. study, the age group of 1-2 years had the highest detection rate for a primary EBV infection [19].
In this study, 27% and 38% of KD patients and age/sex matched controls had positive PCR test of EBV, respectively. In the Kikuta H et al. study, 60% of KD patients had EBV genome within 2 weeks after the onset of KD [20]. In contrast, among 17 control DNA samples,only 12% were polymerase chain reaction positive [10]. In another study on a two year old boy, concurrent KD disease with a primary EBV infection suggested the probability of EBV as an etiologic agent related to the KD [11]. In Marchette NJ et al. study, 22% of KD patients and 45% of controls were anti-VCA IgG-positive and this difference was significant [16].
In Okano M et al. study, 41% and 83% of KD and control groups were seropositive, respectively [15]. In another Korean study, therewas no difference in the rate of positive serology for antibodies against EBV compared to control group [9]. Several studies conducted in Japan reported that the incidence of the EBV antibody in KD patients and in children with a past history of KD is meaningfully lower than that of age-matched control children [12,13,15,20,21]. Our study similar to other serologic studies indicates that EBV is not the pathologic agent of KD [9,16,17].
In conclusion, no association was found between KD and EBV and KD children seem to be infected with EBV similar to normal children of the same age.
References
- Kuo HC, Yang KD, Chang WC, Ger LP, Hsieh KS (2012) Kawasaki disease: an update on diagnosis and treatment. Pediatr Neonatol 53: 4-11.
- Onouchi Y (2012) Genetics of Kawasaki disease: what we know and don't know. Circ J 76: 1581-1586.
- Culora GA, Moore IE (1997) Kawasaki disease, Epstein-Barr virus and coronary artery aneurysms. J Clin Pathol 50: 161-163.
- Huang SK, Lin MT, Chen HC, Huang SC, Wu MH (2013) Epidemiology of Kawasaki disease: prevalence from national database and future trends projection by system dynamics modeling. J Pediatr 163: 126-131.
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, et al. (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110: 2747-2771.
- Mohammadi F, Nadji SA, Karimi S, Mansoori SD, Sharifkashani S, et al. (2006) Detection of Epstein Barr virus DNA in thymic epithelial tumor using nested PCR. Tanaffos 5: 9-13.
- Cohen JI (2000) Epstein-Barr virus infection. N Engl J Med 343: 481-492.
- Hjalgrim H, Friborg J, Melbye M (2007) The epidemiology of EBV and its association with malignant disease. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, et al. (Eds) Human herpesviruses: Biology, therapy, and immunoprophylaxis 53: 403-433.
- Lee SJ, Lee KY, Han JW, Lee JS, Whang KT (2006) Epstein-Barr virus antibodies in Kawasaki disease. Yonsei Med J 47: 475-479.
- Kikuta H, Nakanishi M, Ishikawa N, Konno M, Matsumoto S (1992) Detection of Epstein-Barr virus sequences in patients with Kawasaki disease by means of the polymerase chain reaction. Intervirology 33: 1-5.
- Kanegane H, Tsuji T, Seki H, Yachie A, Yokoi T, et al. (1994) Kawasaki disease with a concomitant primary Epstein-Barr virus infection. Acta Paediatr Jpn 36: 713-716.
- Iwanaga M, Takada K, Osato T, Saeki Y, Noro S, et al. (1981) Kawasaki disease and Epstein-Barr virus. Lancet 1: 938-939.
- Kikuta H, Matsumoto S, Osato T (1991) Kawasaki disease and Epstein-Barr virus. Acta Paediatr Jpn 33: 765-770.
- Kikuta H, Taguchi Y, Tomizawa K, Kojima K, Kawamura N, et al. (1988) Epstein-Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature 333: 455-457.
- Okano M, Hase N, Sakiyama Y, Matsumoto S (1990) Long-term observation in patients with Kawasaki syndrome and their relation to Epstein-Barr virus infection. Pediatr Infect Dis J 9: 139-141.
- Marchette NJ, Melish ME, Hicks R, Kihara S, Sam E, et al. (1990) Epstein-Barr virus and other herpesvirus infections in Kawasaki syndrome. J Infect Dis 161: 680-684.
- Rowley AH, Wolinsky SM, Relman DA, Sambol SP, Sullivan J, et al. (1994) Search for highly conserved viral and bacterial nucleic acid sequences corresponding to an etiologic agent of Kawasaki disease. Pediatr Res 36: 567-571.
- Pasic S, Minic A, Djuric P, Micic D, Kuzmanovic M, et al. (2006) Fever of unknown origin in 185 paediatric patients: a single-centre experience. Acta Paediatr 95: 463-466.
- Huang Y, Wei C, Zheng K, Zhao D (2013) The impact of serological features in Chinese children with primary or past Epstein-Barr virus infections. Virol J 10: 55.
- Kikuta H, Mizuno F, Osato T, Konno M, Ishikawa N, et al. (1984) Kawasaki disease and an unusual primary infection with Epstein-Barr virus. Pediatrics 73: 413-414.
- Okano M, Thiele GM, Sakiyama Y, Matsumoto S, Purtilo DT (1990) Adenovirus infection in patients with Kawasaki disease. J Med Virol 32: 53-57.