Journal of Food Processing & Beverages
Download PDF
Research Article
*Address for Correspondence: Kris Arvid Berglund, Department of Food Science & Human Nutrition, Michigan State University, East Lansing, MI 48824, USA, Tel: +15179743030; E-mail: berglund@msu.edu
Citation: Jeffery JD, Berglund KA. Extraction of Wood Constituents from Non-Conventional, Small Whiskey Barrels. J Food Processing & Beverages. 2016;4(1): 7.
Copyright © 2016 Berglund KA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 4, Issue: 1
Submission: 13 September, 2016 | Accepted: 07 October, 2016 | Published: 17 October, 2016
An emerging artisan, craft industry has begun to grow in the U.S. and other whiskey producing countries. One of the outgrowths of this new industry has been the use of alternative methods that attempt to produce spirits that are of equal quality but require less time for maturation than the traditional products. This interest is driven by the pressure from the start-up capital requirements in the building of a distilling operation and the length of time required before a matured spirit may be bottled and sold.
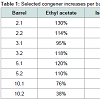
In the case of ethyl acetate a much more drastic change in concentration is apparent. For this study it was not possible to quantify acetic acid as it eluted during sensor acquisition delay.Acquisition delay was necessary because some volatile components (ethanol, amyl alcohols, acetic acid) were present in such high concentrations that they had the effect of overwhelming the sensor and reducing sensitivity to compounds eluting later in relatively lower concentrations. With both ethanol and acetate in this high concentration category, the increase in ethyl acetate is explained by the presence of ample substrate for the production of the resulting ester. While this is a likely explanation, no clear trend is apparent that relates to barrel size, although the greatest increase appears in a 2 gallon barrel while the least increases appear in the 10 gallon barrels.The variation in methanol is not so easily explained (Figures 2-9). In all but barrels 5.2 and 10.2 the trends are remarkably similar, with a 3-4 fold increase beginning at day 84 and returning to original levels by day 202. Ordinarily high concentrations of methanol in fruit spirits are due to demethylation processes related to structural polymerssuch as cellulose in fermentation. General increase trends in congener content during barrel ageing are attributed to evaporation and concentration. While lignolytic activity of ethanol is reported in the literature, lignin is not methylated as heavily as cellulose. Cellulosic ethanolysis has been reported causing increases in carbohydrate concentration of whiskey spirits, which might be the cause of the increases in methanol concentration at certain points during tracking as cellulose is heavily methylated and oak wood is approximately 50% cellulose.In this study the concentration eventually returned to almost its starting point. It is unknown in this case what the mechanism of change might be, unless methyl esters were produced toward the end of the aging period consuming methanol and lowering apparent concentrations. It is also possible that more methanol diffused out of the barrel as its concentration rose. Its molecular weight (MW) being 32 falls between ethanol (MW:46) and water (MW:18). Further examination of such trends would be invaluable for the understanding of the ageing process.
Absorbance
The upward absorbance trend does not follow the same pattern of increase as was observed in the oak extraction rates in 2 and 3 gallon barrels (Figures 10-14). It is unknown what components within the spirit are responsible for the absorbance. Thousands of aromatic substances have been isolated from whiskeys any or all of which might be responsible for the effect, and more data would be required to ascertain of what value this data might be in production.
Barrel extraction
Most published extraction data has been collected from the Scotch whiskey industry which utilizes barrels previously used for the ageing of bourbon whiskey. This means that many of the compounds expected in American whiskeys will not appear in Scotch or Irish as they have been extracted in previous use and their extraction profiles cannot be compared. Oak extraction data is presented in Figure 15. Additionally, because the standards of identity for some spirits allow for blending with neutral spirits, blending of spirits aged for a variety of periods in barrels and activated carbon treatments for the removal of certain compounds, even within the category of American whiskies it is difficult to determine the value of such comparisons. Each of the references below characterized whiskeys aged in 55 gallon barrels.
Extraction of Wood Constituents from Non-Conventional, Small Whiskey Barrels
John D. E. Jeffery1 and Kris Arvid Berglund1-3*
- 1Department of Food Science & Human Nutrition, Michigan State University, East Lansing, MI 48824, USA
- 2Division of Chemical Engineering, Luleå University of Technology (Luleå Tekniska Universitet, LTU), SE-971 87 Luleå, Sweden
- 3Department of Chemical Engineering & Materials Science, Michigan State University, East Lansing, MI 48824, USA
*Address for Correspondence: Kris Arvid Berglund, Department of Food Science & Human Nutrition, Michigan State University, East Lansing, MI 48824, USA, Tel: +15179743030; E-mail: berglund@msu.edu
Citation: Jeffery JD, Berglund KA. Extraction of Wood Constituents from Non-Conventional, Small Whiskey Barrels. J Food Processing & Beverages. 2016;4(1): 7.
Copyright © 2016 Berglund KA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Food Processing & Beverages | ISSN: 2332-4104 | Volume: 4, Issue: 1
Submission: 13 September, 2016 | Accepted: 07 October, 2016 | Published: 17 October, 2016
Abstract
In the aging of whiskey spirits, oak barrels provide vessels for containment which are semi-permeable to the spirit and to outside air. The barrel wood itself provides extractives derived from structural polymers which have been degraded during barrel construction. These extractives have a large cumulative effect on the sensory characteristics of the aging spirit. The traditional barrel for the aging of spirits ranges from 52-60 American gallons and has been employed in the same basic size and form for centuries. Recent growth in themerican craft distilled spirits industry has increased the use of reduced volume barrels ranging from 2-30 American gallons. These smaller barrels provide more rapid extraction and to some extent more rapid oxidation and maturation. While a good deal of research has addressed the topics of extractive concentrations and reactions in the traditional industry, very little information is available on reduced volume barrels. The current study by tracks rates of extraction from 2,3,5 and 10 gallon barrels over the course of 200 days. Four phenolic compounds were chosen for monitoring by gas chromatography coupled with mass spectrometry detection (GC/MS). The results indicate very high rates of extraction to the higher surface area to volume ratios of the smaller, non-conventional barrels.Keywords
Barrel aging; Whiskey; Congeners; Barrel extractivesIntroduction
The traditional barrel used for aging ranges from 52-59 gallons. The surface area to volume ratio of this sized barrel is calculated at roughly 90 cm2/Liter [1]. This number, however, takes only the surface into account, and underestimates the three dimensional migration into and out of barrel depth. The barrel-spirit interface is the site of extraction of phenolic components, and migration of ethanol and water out of the barrel and it has been theorized that the maturation process might be accelerated in smaller barrels [1-3].Work conducted by Conner et al. showed that Scotch whiskey aged in six liter barrels not only extracted phenolic components at a faster rate, but that the spirit itself showed markers of aging in less time. It was theorized that the reduced maturation time was due to increased wood extract and greater oxidation by higher exposure to oxygen due to greater head space in the barrel. This was in part due to the fact that greater evaporation takes place in smaller barrels but also because large sample volumes drawn from the small barrels produced reduced fill volume more quickly than natural migration from the barrel would have [4]. Analytical methods employed in the study required large sample volumes and conditions in the small barrels were affected. It was concluded that smaller casks were inappropriate for the Scotch whiskey industry. It is important to note that casks utilized in this case were not heat treated and it is known that raw oak is not ideal for aging spirits [5,6]. This study is the only published work on alternative barrel sizes to date.
Migration of oxygen into the barrel is an integral component in production of mature spirits, fueling important oxidation reactions, and it has been determined that migration occurs at what is referred to as the ullage, or the headspace above the spirit [1,4]. As evaporation occurs and the fill level decreases, oak not in contact with liquid spirit dries, contracts, and becomes more porous to the entry of outside air [1]. Oxygen present in the barrel fuels oxidation reactions that are essential in the production of mature spirits. It is for this reason that spirits barrels are rarely “topped off” as are wine barrels. Additionally the concentration of flavors that is the consequence of evaporation is an important factor in maturation. Average volume loss from traditional 52-55 gal barrels is 5% per year [1,4,7].
While much work has been conducted in the evaluation of aging and maturing effects in oaked spirits, it has failed to produce quantifiable quality standards as many components are present in very low concentrations and indeed many act synergistically to produce combined flavor effects that are not easily quantified. In fact the presence of low concentrations of a variety of phenolic extractives often produce flavor effects that are as pronounced as those from relatively higher concentrations of, for example, fatty acid ethyl esters. These factors combine to create great difficulty in producing so called quality standards for oaked spirits [4,8-10].
It is however, known that the presence of certain components is necessary for their individual contributions to flavor and through concentrations sufficient to support reactions. Extractives that have in past work been quantified and examined for sensory threshold have been established in a variety of studies to be important to the finishing of spirits [4,6,8,9,11]. For the purposes of this study, four of these components (vanillin: 4-hydroxy-3-methoxybenzaldehyde, eugenol: 4-Allyl-2-methoxyphenol, guaiacol: 2-Methoxyphenol, and 2-Methoxy-4-methylphenol) have been chosen for examination (Figure 1). Substances were chosen based on data present in past work.
It is the objective of this study to begin to develop an understanding of oak extraction rate as it relates to surface area to volume ratio (SA/V), and to obtain this understanding with typical industrial processes. The artisan industry has not established itself yet as an institution and therefore has limited resources with which to fund research and development and is employing production techniques about which little is known.
The traditional size of spirits barrels ranges from 52-59 gallons. In the currently growing artisan spirits industry many producers have taken to the use of smaller barrels and other alternatives for faster extraction through higher surface area to volume ratio, and potentially more rapid aging. While a body of research exists examining the processes of aging in traditional barrels, little information is available examining conditions in non-traditional barrels and little or no information is available on the actual extraction rates of smaller barrels for specific oak components. The current study was undertaken to quantify the rate of extraction of: guaiacol, eugenol, 2-methoxy-4- methylphenol, and vanillin from 2,3,5 and 10 gallon barrels over the ourse of 200 days. Duplicates of each barrel size were employed.
Materials and Methods
Whiskey and barrelsWhiskey was produced from a mash of 79% corn and 21% wheat, mashed and distilled using typical industry practices and then proofed to 62% alcohol by volume before filling the barrels.
American oak barrels were purchased from a well recognized supplier to the artisan spirits industry. The barrels chosen were level 3 char, often employed in the production of American whiskey spirits. The barrels were filled with water at 70 °C, before spirits were introduced to ensure no leakage of spirits, as recommended by the manufacturer. Duplicates of 2,3,5 and 10 gallon barrels were filled on June 5, 2010 and stored at environmental temperatures in a nontemperature controlled warehouse, again as is commonly done in industry. Barrels are named by size and replicate; e.g. Barrel 2.1: 2 gallon barrel, replicate 1; Barrel 3.1: 3 gallon barrel, replicate 1; Barrel 10.2: 10 gallon barrel, replicate 2, etc.
The barrels were sampled every week for the first month and then sampling time was reduced to monthly. Analysis is presented here for a 200 day sampling duration. For sampling, two milliliters were removed from each barrel, held in GC vials at refrigeration temperature and analyzed when equipment availability allowed. In an attempt to minimize variation in the barrels, samples were removed after gentle agitation. Temperatures were recorded but not controlled. Final volumes and % ABV (alcohol by volume) were measured to establish evaporation rate.
Analysis
For analysis of volatile congener concentrations, a Shimadzu gas chromatograph with auto-injector and flame ionization detector (FID) was utilized. An Agilent Stabilwax column with fused-silica capillary column, 30 M long, with 0.25 mm inner diameter and 0.25 um film thickness was injected with 0.4 uL injection with 20:1 split,held at 33 °C for 1 min and then temperature increased at 9/min to 110 °C, held for 1 min and reduced to 33 °C. Helium was used as a carrier gas. External standards were prepared and a standard curve produced which was used to calculate concentrations. Standards were run monthly to ensure that changes in column response were taken into account. This method yielded quantitative analysis of volatile components.
For analysis of oak extractives a GC was utilized with an autoinjector, and linked MS in electron ionization (EI) mode. An Agilent DB-Wax column with the same liner and dimensions as those listed for the GC was injected with 5 uL splitless injection, held at 40 °C for 5 min, increased at 5/min to 100 °C, and then at 2/min to 210 °C, held for 45 min and cooled to 40 °C. Helium was used as carrier gas with1.5 mL/min initial flow, 11.93 psi pressure, and average velocity of 44 cm/sec. The injector was held at 250 °C and the GC to MS transfer line was held at 240 °C. Purge flow of 50.0 mL/min was held for 2.0 min. A 14.5 min solvent delay (data acquisition delay) was necessary as the initial volatile components (ethanol and volatile congeners) had the effect of decreasing the total sensitivity of the detector during the run, due to the large injection volume, and high concentrations. The ion signals were detected over the range of 10-350 and pre-identification of components was performed with the National Institute of Science and Technology (NIST) mass-spectral library. These identifications were confirmed using external GC grade standards purchased from Sigma Aldritch.
Standard curves were produced with the external standards and used to calculate concentration from peak areas. Standard solutions were mixed using each analyte individually in 62% ABV and water (the concentration at which the barrels were filled). A standard was mixed using 20 mg/l of each analyte and a 5 part dilution series wasconducted yielding concentrations of 20 mg/l, 2 mg/l, 0.2 mg/l, 0.02 mg/l, and 0.01 mg/l. The dilution procedure was conducted in triplicate. A four point standard curve was produced using the initial dilution set and then confirmed using the subsequent two sets. Where any variation above 0.5% occurred standards were remixed. All standards were stored below 0 °C, and the 0.02 mg/l standard was rerun before analysis of any samples, to confirm column response.
While common practice in industry for GCMS analysis of oak extractives employs extraction and concentration of phenolic components before analysis, this was not utilized as it would have required larger sample volumes (10-1000 ml). In previous research this approach was used to increase peak separation. It was decidedthat because larger sample volumes would significantly affect the SA/V ratio by decreasing the spirit volume during aging, and therefore artificially decreasing extraction, that a large volume injection combined with a relatively slow temperature profile would be utilized to produce the necessary peak separation and allow for smaller sample volumes (2 ml). A method was adapted from the literature to minimize the sample volume and hence the effect of sampling on volume and therefore spirit/barrel interactions. Compounds were chosen for analysis by consistent peak separation and good quality peaks.1- A glass cuvette (25x25x20 mm)
A Perkin-Elmer Lambda 3A model spectrophotometer was utilized to collect absorbance data. Plastic cuvettes with a 1 cm path length employed as the compounds of interest were in the visible range. Absorbance was collected with the unaged distillate in the reference cell. Unaged distillate was stored in pyrex containers in darkness. Two milliliters of whiskey were removed from each cask, measured and returned to the barrel.
An Anton Paar DM 5000 densitometer was used for analysis of % ABV at the beginning and end of the study.
Results and Discussion
Concentrations of some volatile congeners, shown in Table 1 increased, in some cases quite drastically. The changes in apparent concentrations of some compounds can likely be attributed to the nature of aging in the barrel. Where decreases in higher alcohols arefound (around day 50) this may be due to the production of esters with fatty acids, where the concentration of the alcohol appears to decrease as it is consumed by reaction. The slight increases toward day 200 are likely due to evaporation of water and ethanol from the barrel and concentration of the remaining compounds in the remaining spirit. These two processes proceed simultaneously as the same evaporation which causes apparent increases, allows more air to penetrate into the barrel fueling production of esters.Absorbance data trended upward for most of the study. Absorbance at A520 has been utilized by MacDougall in the development of a uniform color scales for whiskies. From the data presented in Figure 10 it is apparent that absorbance may be valuable for tracking barrel extraction. This might take the form of empirical data collected and employed in QAQC facilities in industry, but may also warrant further examination in a laboratory setting. This methodology for barrel tracking has not been suggested in the literature or examined for its utility. With the prevalence of affordable and portable spectrophotometric devices this could become a valuable technology in the production setting.
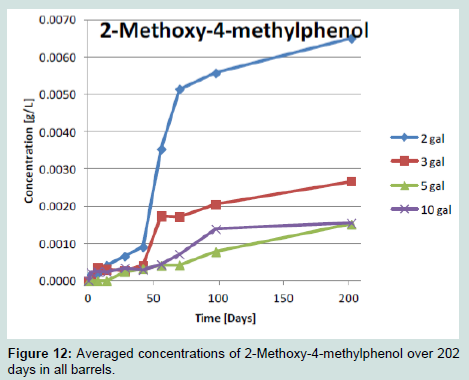
For each of the compounds analyzed extraction occurred faster at smaller barrel volumes. One interesting phenomena noted was a latent period in extraction during the first 40 days in 2 and 3 gallon barrels. Between days 40 and 60 the 2 and 3 gallon barrels displayed a rapid increase in concentration of each phenolic compounds. It is unknown why the smaller barrels would display this pattern but it is also interesting to note that after this period of rapid extraction the flavor of the spirit could be considered over extracted with the flavor of oak in these spirits becoming overwhelming. The 5-10 gallon barrel roduced a more gradual and consistent increase in concentration during the full 202 days, for all compounds, with no latent period.
It is a known feature of oak ageing that spirit migrates into and out of the wood staves. Migration of liquid into the barrel wood and back into the container with solubilized phenolic constituents is the method of extraction. The barrel stave thickness becomes slightly greater as the barrel volume grows and it is possible that the smaller staves in the 2 and 3 gallon barrels were affected differently by heat treatment causing the change in extraction activity. Heat treatment is an uncontrolled factor in this study and so its affect on the available extractive content.
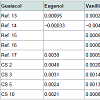
In the construction of a 55 gal barrel, staves are produced from many trees and potentially multiple subspecies of Quercus and the average 50 gallon barrel is constructed of 55 staves. This random sampling of staves has the effect of minimizing specific effects from differing growth conditions of the individual trees on the sensory qualities of the spirit. Because there are fewer staves utilized in theconstruction of smaller barrels there is more opportunity for the effects of an individual growth condition to influence the extraction profile of the whiskey. Additionally in the large production setting whiskeys are blended from many barrels, again reducing the impact of individual growth factors on the sensory qualities of whiskeys. The artisan producer does not have access to this large volume of aged product for blending and may be subject to greater impact from individual trees on the sensory qualities of their products.Table 2 shows final concentrations from this study and as collected in previously published research. Very few studies exist which display concentrations of multiple compounds extracted from new American oak in American whiskeys. These concentrations were comparable in some cases and far higher in others to those values found in the literature. Again, this points to the lack of data in this area of research and the need for further data.
Table 2: Comparison of phenolic components [g/l] as reported in previous work and in the current study (CS) in 2,3,5, and 10 gallon barrels (CS 2, CS 3, CS 5, CS 10, respectively). Ref. 13- a 3 yr bourbon; Ref. 14- 10 yr Scotch; Ref. 15- American bourbon; Ref. 16- American whiskey; Ref. 17- commercial rye whiskey.
Conclusion
The current study has begun the process of characterization of extraction from non-traditional barrel sizes. This represents a starting point for an area of research which should be considered useful to a segment of the American distilled spirits industry that utilizes these barrels to produce spirits rather than or in addition to traditional barrels.It may be said of the extraction data, that it is confirmed that extraction rate is coupled to SA/V ratio, and that faster and sometimes greater extraction is possible as SA/V increases. Guaiacol concentrations of compounds were as much as 100 times higher than those found in some literature, and comparable to others [12-16]. Eugenol concentrations were 10 times higher than those found in some literature and slightly less than double those found in others [12-16]. Vanillin concentrations were comparable to some at the 10 gallon size and comparable to others at the 2 gallon size [12-16].
This not only points to the great variation in concentration between styles of whiskey, it also emphasizes the great variation between studies and the difficulty with standardization of such compounds for whiskey spirits. While it is clear that smaller barrels lead to faster extraction and in some cases higher concentrations it is difficult to establish target concentrations. A larger body of knowledge is available for the scotch whiskey industry which utilizes barrels which have been used once for the aging of bourbon. Used barrels lead to lower concentration of some oak extractives and therefore are not as useful in the context of the American artisan whiskey industry.
Further study would be useful for the elucidation of the total aging process in alternative barrels. For this, the use of a concentration and extraction method might be necessary to examine concentrations on a longer time scale. Because of the large angel’s share from 2 and 3 gallon barrels they must be considered unfeasible for use in industry for anything other than rapid examination of certain oak characters in trial whiskies. As such, barrels of 10,20 and 30 gallons might be examined for extraction and aging character and in the context of the larger barrels, larger samples appropriate to extraction and concentration would be possible. It would be useful to compare these in the laboratory setting to conditions in traditional barrels, however this becomes problematic as the time required to conduct such studies is measured in years. This is the primary reason such research has not been completed. In past studies samples are taken from bottles of commercial products or from barrels within the commercial setting. This means that the researchers had little control over the ageing process and sampling was not conducted over time within individual barrels.
To further elucidate the total aging process it would be advisable to track production of esters during aging. It was noted that volatile congeners increased and decreased at different points in the aging process. While it may be posited that this was coupled to ester production and evaporation processes it would be useful to track these processes more closely to determine whether this was the case. This would provide a more coherent understanding of processes as they relate to one another.
In addition to the above suggestions, sensory data coupled to extraction rate and concentration would be helpful. It was noted that the 2 and 3 gallon barrels were over extracted and their sensory qualities were poor. Other barrels in the series tasted over extracted at certain points but by the end of the study at day 270, both 5 and 10 gallon barrels had developed some characteristics of mature whiskies and their sensory qualities had begun to improve. While this is a subjective judgment of the author, samples were compared to industry products and found to have some qualities in common that had been lacking at previous points. This is an important feature as it points to the fact that while extraction is an important process, time is required to incorporate the extracted compounds into the spirit to produce maturity. It is also important to note that while over extraction is possible, even higher than normal concentrations of extractives may be fully integrated into the spirit given enough time.
With further examination it might be possible to establish a spectrophotometric procedure to allow producers to estimate total extraction during the aging period. This might provide a quick standard by which a variety of barrels might be compared for blending purposes. Affordable spectrophotometric units are now available which could be employed in a production setting for tracking of extractives. This might be linked to particular markers of aging such as ethyl esters and oak extractives and used to supplement current techniques which rely largely on sensory analysis.
Acknowledgements
The authors recognize the MSU Mass Spectrometry Facility for use of the GC/MS instrumentation used in this study. Financial support was supplied by MSU AgBioResearch.References
- Singleton VL (974) Some aspects of the wooden container as a factor in wine maturation. In: Webb A (Ed). Chemistry of winemaking. Advances in chemistry. American Chemical Society, Washington, DC.
- Conner J, Reid K, Jack F (2003) Maturation and blending. In: Russel I (Ed). Whisky: technology, production and marketing, Academic Press, London, UK, pp. 211-234.
- Philip JM (1989) Cask quality and warehouse conditions. In: Piggott JR, Sharp R, Duncan RE, (Eds). The science and technology of whiskies. Longman Scientific and Technical, Essex, England.
- Withers JS, Piggott JR, Connor JM, Paterson A (1995) Comparison of scotch malt whisky maturation in oak miniature casks and American standard barrels. J Inst Brew 101: 359-364.
- Martinez RG, Serrana HL, Mir MV, Granados JQ, Martinez MC (1996) Influence of wood heat treatment, temperature and maceration time on vanillin, syringaldehyde, and gallic acid contents in oak wood and wine spirit mixtures. Am J Enol Vitic 47: 441-446.
- Wildenradt HL, Singleton VL (1974) The production of aldehydes as a result of oxidation of polyphenolic compounds and its relation to wine aging. Am J Enol Vitic 25: 119-126.
- Reazin GH (1981) Chemical mechanisms of whiskey maturation. Am J Enol Vitic 32: 283-289.
- Lee KY, Paterson A, Piggott JR, Richardson GD (2001) Origins of flavor in whiskies and a revised flavour wheel: a review. J Inst Brew 107: 287-313.
- Mosedale JR, Puech JL (1998) Wood maturation of distilled beverages. Trend Food Sci Tech 9: 95-101.
- Salo P Nykanen L, Suomalainen H (1972) Odor thresholds and relative intensities of volatile aroma components in an artificial beverage imitating whisky. J Food Sci 37: 394-398.
- Viriot C, Scalbert A, Lapierre C, Moutounet M (1993) Ellagitannins and lignins in aging of spirits in oak barrels. J Agric Food Chem 41: 1872-1879.
- Poisson L, Schieberle P (2008) Characterization of the key aroma compounds in an American bourbon whisky by quantitative measurements, aroma recombination, and omission studies. J Agric Food Chem 56: 5820-5826.
- MacNamara K, van Wyk CJ, Brunerie P, Augustyn OP, Rapp A (2000) Flavour components of whiskey. III. Ageing changes in the low-volatility fraction. S Afr J Enol Vitic 22: 82-92.
- Goldberg DM, Hoffman B, Yang J, Soleas GJ (1999) Phenolic constituents, furans, and total antioxidant status of distilled spirits. J Agic Food Chem 47: 3978-3985.
- Lehtonen M (1982) Phenols in whisky. Chromatographia 16: 201-203.
- Kahn JH, Shipley PA, LaRoe EG, Conner HA (1969) Whiskey composition: identification of additional components by gas chromatography-mass spectrometry. J Food Sci 34: 587-591.