Journal of Veterinary Science & Medicine
Download PDF
Research Article
Study on Prevalence of Helminthes of Local Backyard and Exotic Chickens in and Around Ambowest Shoa Zone, Oromia Regional State, Ethiopia
Ali Solomon Shiferaw*, Firaol Tamiru, Askale Gizaw, Dagmawit Atalel, Waktole Terfa, Morka Dandecha and Abreham Mekibib
- Department of Veterinary Laboratory Technology, College of Agriculture and Veterinary Science, Ambo University, Ambo, Ethiopia
*Address for Correspondence: Solomon Shiferaw, Department of Veterinary Laboratory Technology, College of Agriculture and Veterinary Science, Ambo University, P.O.Box 19, Ambo, Ethiopia, E-mail: solbruk40@yahoo.com
Citation: Shiferaw S, Tamiru F, Gizaw A, Atalel D, Terfa W, et al. Study on Prevalence of Helminthes of Local Backyard and Exotic Chickens in and Around Ambowest Shoa Zone, Oromia Regional State, Ethiopia. J Veter Sci Med. 2016;4(2): 4.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 4, Issue: 2
Submission: 22 August, 2016| Accepted: 17 December, 2016 | Published: 23 December, 2016
Copyright: © 2016 Shiferaw S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
A cross sectional study was conducted on local and exotic chickens in and around Ambo district of west show a zone Oromia regional state, Ethiopia, to determine the prevalence of gastrointestinal helminthes infections and identify the involved parasitic species. A total of 265 local and 125 exotic chickens were purchased, scarified and their gastro intestinal tracts were examined for adult helminthes. The overall helminthes prevalence was 68.5% and mixed helminthes infections were found in 33% of birds. The study also found that 191 (49%) and 176 (45.1%) of chickens was infected by diverse species of nematodes and cestodes species, respectively. Four nematodes species were identified in the prevalence rate of Ascaridia galli (72.25%), Heterakis gallinarum (44.5%), Capillaria obsignata (5.23%) and Syngamus trachea (1.04%). The major cestodes species encountered were Rialletina tetragona (50%), Davenia proglotina (31.8%), Choanotaenia infundibulum (0.57%), Rialletina echinobothrida (11.93%), Amoebotaenia cuneata (1.13%) and Rialletina cesticillus (0.57%). There was statistically significant differences (p<0.05) in the prevalence between breeds of chickens in which higher infection was observed in local breed (78.11%) than exotic breed (48%). There was also a statistically significant differences (p<0.05) in prevalence rate between the different management systems where there was higher infection was observed in extensive management system (78.54%) compared to semi intensive management system (33.35%). The study also tried to see the prevalence of these parasites in relation with age and sex however, there is no significant differences (p>0.05) with this risk factors. This study strongly suggested that helminthes parasites are a very serious problem of local chickens in the study area and appropriate control and prevention strategies need to be applied.
Keywords
Ambo; Cestodes; Chicken; Ethiopia; Nematode; Prevalence
Introduction
Chicken, Gallus gallus is believed to have descended from the wild Indian and South East Asian red jungle fowl [1]. The bird provides man with high nutritional value and other socioeconomic benefits which cannot be overemphasized [2]. Besides providing employment and income for small-scale farmers particularly in the off cropping season, poultry integrates very well into other farming activities like cropping and fish farming [3].
Approximately 20 billion poultry exist worldwide [4] and of this about 75% are in developing countries. Village chickens (Gallus gallus) are the predominant species in the rural poultry sector in Africa [4,5] and in Ethiopia total poultry population is estimated to be 56.5 million [6] of which about 99% are raised under the traditional backyard system of management, while 1% are exotic breeds maintained under intensive management system.
Poultry production system in Ethiopia is an indigenous and integral part of the farming system that ranges from nil input traditional free ranges to modern production system using relatively advanced technology. There is also a small-scale intensive system with small number of birds (from 50 to 500) as an urban and periurban small-scale commercial system using exotic birds and relatively improved feeding, housing and health care [7,8]. The backyard (traditional) poultry production system is characterized by low input, low output and periodic destruction of large proportion of the flock due to disease outbreaks [9].
A lot of losses in poultry have been linked to disease causing agents such as viruses, bacteria and parasites [10]. Although parasitic diseases are among the major causes that decrease productivity of chickens, they are often neglected as they are rarely lethal. Helimnthosis was considered to be an important problem of local chickens and helminth parasites were incriminated as major causes of ill-health and loss of productivity in different parts of Ethiopia [6]. Helminth parasites of poultry are commonly divided into three main groups; nematodes, cestodes and trematodes. Nematodes constitute the most important group of helminth parasites of poultry both in number of species and the extent of damage they cause; the main genera include Capillaria, Heterakis, and Ascaridia. The cestodes of significant importance are of the two genera Railleitina and Hymenolepsis [11]. In the commercial table egg production systems the most commonly occurring helminth species are Ascaridia galli, Heterakis gallinarum and Capillaria spp. [12].
The prevalence and intensity of helminth may be influenced by several factors, including host factors, such as age, sex and breed, can also influence helminth infections. Furthermore, climatic conditions (temperature and humidity) may alter the population dynamics of the parasites, resulting in dramatic changes in the prevalence and intensity of helminthes infections [13]. Many insects that may act as vectors for helminthes are also favored by high temperatures and to some extent humidity. These factors may explain the wide range and distribution of nematode and cestode species in poultry, especially during the tropical rainy season [14].
There are currently little information in Ethiopia that shows the prevalence and distribution of gastrointestinal tract (GIT). According to reports, the prevalence of GIT parasitism reaches 91.01% [6,15]. But it is limited by its coverage (region) of Ethiopia so that does not indicate the whole picture of the prevalence in Ethiopia. This was the rationale that initiated this research project. Therefore the objective of this study were to determine the prevalence of helminthes infection and to identify different helminthes parasites in chickens of local and exotic breeding West show a Administrative Zone of Oromia, Ambo district, Ethiopia.
Materials and Methods
Study area
The study was conducted from July to October, 2015 in and around Ambo town, West Shewa zone, Ethiopia. Ambo town is administrative center of West Shewa zone and Ambo district, and located at a latitude and longitude of 8º59‘N 37º51‘E 8.983ºN 37.85ºE and an elevation of 2101 meters above sea level (ASL) and 114 km west of Addis Ababa, capital of Oromia region and Ethiopia. The agro-ecology of the study area is 23% highland, 60% midland, and 17% lowland. It has an annual rainfall and temperature ranging from 800-1000 mm and 20-29 ºC, respectively. The livestock population of the district includes 145371 cattle, 50152 sheep, 27026 goats, 105794 chickens, 9088 horses, 2914 donkeys and 256 mules [16].
Study animals
Apparently healthy local chickens n = 390, (241 females and 149 males) were randomly bought from local open air markets in the respective study areas. The chickens were then transported to Ambo University, Department of Veterinary Laboratory Technology Laboratory. The birds were categorized into two age groups, namely growers and adults. The age of birds was determined subjectively based on the size of crown, length of the spur, and appearance of the beak and flexibility of the xyphoid cartilage [17].
Study design
Cross-sectional type of study design was used for this study. Sex, different age groups, breeds and management systems were recorded as test variable during data collection of target chickens.
Methodology
Thorough clinical examination of each chicken was performed. The chickens were divided into two categories such as local backyard and exotic Chickens and then euthanized and evisceration was undertaken. The alimentary canal was separated from the other organs and removed from the body cavity. The alimentary canal from each chicken was then opened, from the esophagus to the rectum, and including both cecal pouches. All worms visible to the naked eye were removed using thumb forceps. All the adult worms were identified directly under the stereomicroscope using the characteristics described by [18,19]. Scrapings were also taken from the mucosae of esophagus, the upper, middle, lower intestine and caecum and examined under the microscope.
Statistical Analysis
Collected data were first entered into a Microsoft Excel spreadsheet and analyzed using SPSS version 20 software. Descriptive statistical analysis was used to summarize and present the data collected. The helminth prevalence was calculated as the number of chickens harbour helminth parasite in their GIT, divided by the total number of birds examined. The degree of association between each risk factor and parasite infection was assessed using the Pearson Chisquare test. For all analyses, a p-value of less than 0.05 was considered as significant.
Result
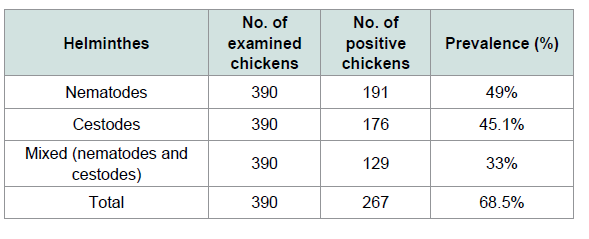
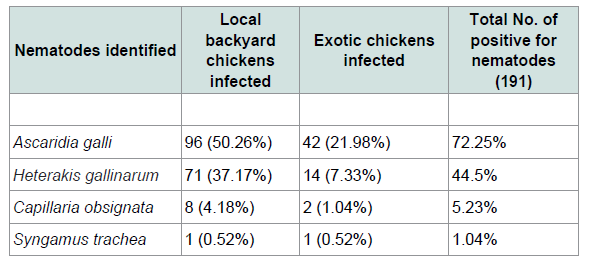
The overall prevalence of infection with helminthes was 68.5% (267/390). Out of 390 chickens 49% (191/390) were found to be infected by nematodes and 45.1 (176/390) were infected by cestodes parasites. While 33% (129/390) had mixed infection Table 1. From 265 local and 125 exotic breeds of poultry birds (Gallus gallus) examined for screening of helminthes parasites (78.11%) local backyard chickens and (48%) exotic chickens were harbored with different species of helminthes (Tables 2 and 3). A total of ten different species of helminthes parasites were isolated and identified. The result revealed that Ascarida galli was the highest prevalence rate of infection in both local and exotic breeds. The percentage prevalence recorded was 50.26% and 21.98% respectively.
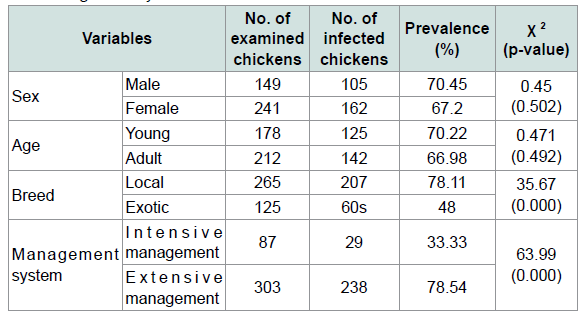
Although helminthes infection was more prevalent in males(70.45%) than females (67.2%) and in young (70.22%) followed by adult (66.98%) statistically there were no significant differences. Additionally in the prevalence of helminthes parasites between sexes and different age groups of chickens there were no significant differences (p>0.05) (Table 4). The prevalence of helminthes was significantly different between breed and different management systems (X2=35.67, 63.99) respectively. An effort was made to identify the species of nematodes and cestodes in chickens in the study area. Consequently, the species of nematodes and cestodes identified were briefly indicated on Tables 2 and 3.
Discussion
The overall prevalence of the current study that was recorded (68.5%) in chicken is lower than that of [20] which is 99%, [15] of 91%, [11] of 87%, [6] of 86.3% and 75.8%, but the present result was slightly higher than that of [21] of 61.9% and [22] of 37.9%. However, the current result is in agreement with the result of [23] which report 64.7%. The present study revealed that nematodes and cestodes parasites were recovered from the chickens. This might be an indication of higher availability of infective stages of the worm in the study area and the ability of the infective stages of the worm to survive outside the host for a long time before it is picked up by the host. Another reason for relatively high prevalence could be especially due to the inability of the farmers to feed the chickens with grains in the morning before they go out for roaming. This could expose the chickens to feed different arthropods that may be carriers of the infective stages of the parasites which have been shown to increase susceptibility to parasitism. The finding of this study is in line with the work of [24] in which nematodes and cestodes were implicated as the major causes of helminthes infection both in young and adult chickens.
There were significant differences in the prevalence rate of helminthes between extensive and semi intensive management systems (p<0.05). This might be chickens kept in extensive management system has unhygienic practices followed with poor nutritional supplies. This result was in agreement with the finding of earlier workers [25-27].
The current prevalence of infection in local breed (78.11%) was significantly higher than the exotic breed (48%) (X2=35.67, p<0.05). This might be due to their free range mode of management practice which allows them free access to virtually all types of environments and hence predisposing them to various forms of infections. Another reason may be the duration for the local breed to reach table size is much longer compared to the exotic breed which feed usually on artificial diets. This cause could be likely the reason for higher infection in the local breed which continue to accumulate parasites in the system as well as the poor management practices inherited in extensive management system. This finding is in line with the previous work by [11], 90.2% and 53% in local and exotic breed respectively; another work done in Nigeria by [28] indicated that the prevalence of helminthes is highly significant in local breed than exotic 87% and 27.3% respectively.
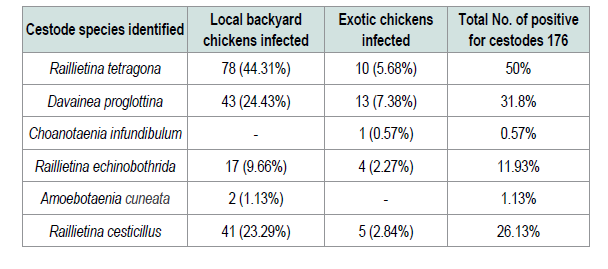
In the present study four nematode parasites, namely Ascaridia galli, Heterakis gallinarum, Capillaria obsignata and Syngamus trachea were identified from different sites of gastrointestinal tract and trachea of examined chickens. Ascaridia galli is the most prevalent species of nematodes that was identified (72.25%) among the intestinal nematodes recovered in the study area. This was higher compared with other finding in Ethiopia and Kenya reported to be 35.58% [15], 55.3% [6] and 33.3% [29]. Additionally previous study in southern Ethiopia [30] reported 64.3% was slightly lower than the current study. This might be wet and humid conditions are necessary for the development of Ascaridia galli eggs to infective stages and the rise of earth worm’s population that serves as a paratenic host for these parasites. The reason for high occurrence of the parasites in the study area is the survey was conducted in wet season. The second most prevalent species of nematodes that was identified in the current study was Heterakis gallinarum (44.5%) which was relatively comparable to prevalence of 43.24% established by [31] the prevalence rate of Syngamus trachea that was found in the current study was low (1.04%). This is in agreement with [21,32] who reported in their works that, this parasite has low prevalence rate of infection compared to other helminthes. As far as cestodes infections were concerned different spices of cestodes were identified. From 390 examined chickens 176 (45.1%) prevalence was obtained. Six species of cestodes were observed. The most prevalent cestodes recorded in and around Ambo was Raillietina tetragona (50%). This observed result is in line with reports of [31,33] with the prevalence rate of 52.75% and 56.5% respectively. However the present result is higher than that of the previous ones reported from different parts of the country [6,11,15,24] with the prevalence of 12.53%, 45.69%, 35.8% and 22.2% respectively. Davainea proglottinais the second most pathogenic species was recorded in the prevalence rate of 31.8%. This result is higher than the previous work done by [15,29,33,35] who reported 8.1%, 1.12%, 5.97% and 19.4% respectively. This might be due to the availability of the intermediate host such as dung beetles, antsetc. in the study area and the environmental conditions which favor the reproduction of intermediate host. Choanotaenia infundibulum is the least cestode encountered in the study area. This is in line with the study of [15]. The mixed infections of two or more species of parasites per bird were very common in the present study. This might be due to food preference at a particular time which determines the establishment of mixed or single infection in the chickens. Mixed infections have been reported in several studies [21,24,36] in current study majority of chickens harbored multiple infections may be due to poor management system.
As a conclusion, the present study clearly indicated that local chickens kept under extensive management system in the study area were highly infected and exposed to wide variety of gastrointestinal helminth parasites. The damage inflicted by such highly prevalent gastro-intestinal helminth infection in view of the economic importance of poultry production, in the study area particularly and in the country generally will undoubtedly be high. It is therefore, recommended that integrated control strategies have to be put in place since there is little effort directed currently towards this problem. In addition, other research works in the areas of poultry gastrointestinal helminths should be conducted in the backyard poultry in order to estimate economic losses inflicted by these parasites.
References
- Permin A, Ranvig H (2001) Genetic resistance to Ascaridia galli in chickens. Vet Parasitol 102: 101-111.
- Matur BM (2002) Prevalence of some gastrointestinal parasites in pullets of chickens (Gallus gallus domestica) in the Federal Capital Territory Abuja, Nigeria Journal of tropical Biosciences 2: 78-82.
- Aini I (1990) Indigenous chicken production in south-east Asia. World Poultry Sci J 46: 51-57.
- FAO (2007) Food and Agriculture Organization Statistical databases. FAO, Rome.
- Kitalyi AJ (1988) Village chicken production systems in rural Africa household food security and gender issues. FAO Animal Production and Health Papers142. Publishing Management Group, FAO Information Division, Rome, Italy.
- Ashenafi H, Eshetu Y (2004) Study on gastrointestinal helminths of local chickens in central Ethiopia. J Vet Med 155: 504-507.
- Mekonnen GM (2007) Characterization of the smallholder poultry production and marketing system of Dale, Wonsho and Loka Abaya Weredas of SNNPRS. Hawassa University, Awassa, Ethiopia.
- Mekonnen S, Berehanu TY, Argaw A (2011) Introduction and evaluation of modified hay-box brooder, Fayoumi chicken and layers housing, addressing small-scale semi-intensive poultry farming at Beresa Watershade, Gurage Zone, Ethiopia. Afr J Anim Biom Sci 6: 102-106
- Alemu S (1985) The status of poultry research and development in Ethiopia. In: IAR proceedings, (Ed). The status of livestock, pasture and forage research and development in Ethiopia, Agricultural Research Institute, pp. 62-70.
- Sayyed R, Phulan M , Bhatti W, Pardehi M, Ali S (2000) Incidence of nematode parasites in commercial layers in Swat. Pak Vet J 20: 107-108.
- Matur BM, Dawam NN, Malann YD (2010) Gastrointestinal helmith parasites of local and exotic chickens slaughtered in Gwagwalada, Abuja (FCT), Nigeria. N Y Sci J 3: 96-99.
- Roy DK (2002) Helminthosis of free-range chickens in Bangladesh - with emphasis on prevalence and effect on productivity.
- Magwisha HB, Kassuku AA, Kyvsgaard NC, Permin A (2002) A comparison of the prevalence and burdens of helminth infections in growers and adult free-range chickens. Trop Anim Health Prod 34: 205-214.
- Dube S, Zindi P, Mbanga J, Dube C (2010) A study of scavenging poultry gastrointestinal and ecto-parasites in rural areas of Matebelel and province, Zimbabwe. Int J Poult Sci 9: 911-915.
- Eshetu Y, Mulualem E, Ibrahim H , Berhanu A , Aberra K (2001) Study of gastro-intestinal helminths of scavenging chickens in four rural districts of Amhara region, Ethiopia. Revue Scientifique et Technique (International Office of Epizootics) 20: 791-796.
- ATMA (2010) Ambo Town Ministry of Agricultural Office: Annual report, Ambo, Ethiopia.
- Damerow G (1995) Storey’s guide to raising chickens. Storey Books, USA.
- Soulsby EJ (1982) Helminths, arthropods and protozoa of domesticated animals, (7thedn). Lea & Febiger.
- Troncy PM (1989) Helminths of livestock and poultry in tropical Africa. In Manual of tropical veterinary parasitology.
- Mwale M, Masika PJ (2011) Point prevalence study of gastro-intestinal parasites in village chickens of Centane district, South Africa. Afr J Agric Res 6: 2033-2038.
- Luka SA, Ndams IS (2007) Gastrointestinal parasites of domestic chickens Gallus-gallus domesticus Linnaeus 1758 in Samaru, Zaria Nigeria. Sci World J 2: 27.
- Dawet A, Yakubu DP, Daburum YH, Dung JP, Haledu UI (2012) Gastrointestinal helminths of domestic chickens (Gallus gallus) in Jos, plateau state, Nigeria. Niger J Parasitol 33: 85-89.
- Tolossa YH, Shafi ZD, Basu AK (2009) Ectoparasites and gastrointestinal helminthes of chickens of three agro-climatic zones in Oromia Region, Ethiopia. Anim Biol 59: 289-297.
- Yoriyo KP, Adarg KL, Adamu SU, Panda SM (2008) Prevalence of gastrointestinal helminthes of free- range chickens and guinea fowls In Bauchi and its Environs. Bull Pure Appl Sci 27: 1-6.
- Hange RR, Raote YV, Jayraw AK (2007) Prevalence of helminth parasites in desifowl (Gallus gallus domesticus) at Parbhani. J Parasit Dis 31: 61-64.
- Katoch R, Yadav A, Godara R, Khajuria JK, Borkataki S, et al. (2012) Prevalence and impact of gastrointestinal helminthes on body weight gain in backyard chickens in subtropical and humid zone of Jammu, India. J Parasit Dis 36: 49-52.
- Sonune MB (2012) Analysis of gastrointestinal parasites of poultry birds around Chikhli, Buldana (M.S.) India. Sci Res Rep 2: 274-276.
- Agbolade OM, Oni TT, Fagunwa OE, Lawal KM, Adesemowo A (2009) Faecal contamination of dump sites in some communities in Ijebu-North, south-western Nigeria. Nigerian J Parasitol 30: 57-60.
- Mungube EO, Bauni SM, Tenhagen BA, Wamael LW, Nziokas SM, et al. (2008) Prevalence of parasites of local scavenging chickens in a selected semi-arid zone of Eastern Kenya. Trop Anim Health Prod 40: 101-109.
- Teshome M (1993) Preliminary survey of gastrointestinal helminthes in local chickens in and around Wolayta Soddo, Addis Ababa University, Ethiopia, pp.18-38.
- Bersabeht (1999) Study of ectoparasites and gastrointestinal helminthes of backyard chickens in three Agro climatic zones in Central Ethiopia. Addis Ababa University, pp.17-44.
- Pam VA, Daniel LN (2006) The survey of intestinal parasites of local and exotic chickens slaughtered at Yankari market, Jos, Plateau State. J Med Pharm Sci 2: 27.
- Heyradin H, Hassen C, Yosef D, Molalegne B (2012) Gastrointestinal helminthes are highly prevalent in scavenging chickens of selected districts of Eastern Shewa zone, Ethiopia. Pak J Biol Sci 15: 284-289.
- Molla WH, Haile G, Almaw, Temesgen W (2012) Gastro intestinal helminths of local backyard chickens in North Gondar Administrative Zone, Ethiopia 7: 362-367.
- Ohaeri CC, Okwum C (2013) Helminthic parasites of domestic fowls in Ikwuano, Abia State Nigeria. J Nat Sci Res 3: 1-5.
- Negesse T (1992) Internal parasites of local chickens of Leku, Southern Ethiopia. Ethiopian J Agric Sci.