Journal of Veterinary Science & Medicine
Download PDF
Research Article
*Address for Correspondence: Panagiota Florou-Paneri, Laboratory of Nutrition, School of Veterinary Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece, Tel: (+30) 2310999968; Fax: (+30) 2310999984; E-mail: ppaneri@vet.auth.gr
Citation: Giannenas I, Skoufos I, Bonos E, Sarakatsianos I, Tzora A, et al. Effect of Dietary Iron Sulfate and Iron Chelate on Growth Performance,Hematological Traits, Intestinal Microbial Flora of Fattening Pigs and Quality Parameters of Porkmeat. J Veter Sci Med. 2015;3(2): 11.
Copyright © 2015 Giannenas I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 3, Issue: 2
Submission: 29 September, 2015 | Accepted: 09 November, 2015 | Published: 14 November, 2015
Animals, experimental design and diets
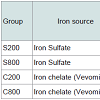
The diet of the other three groups, was the same with the only difference that it contained per kg either 800 mg iron sulfate (Group S800), or 200 mg iron chelate (Group C200), or 800 mg iron chelate (Group C800). The dietary iron fortification of the different groups is presented in Table 2.
At the end of the trial (165th days of age), all animals were individually weighted and slaughtered in a commercial slaughter house and samples were taken for further analyses.
START D Microwave digestion system (Milestone Srl Sorisole (BG) - Italy) was used for sample preparation. Processed meat samples were homogenized by Ultra-Turrax type Yellow line by IKA DI 18 Basic Homogenizer (IKA Werke GmbH & Co., Staufen, Germany) at 5,000 rpm for three min.
Blood traits
Enumeration of intestinal microbiota
Carcass quality characteristics
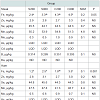
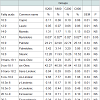
Meat major and trace element analysis: The concentrations of trace elements in steak, ham and shoulder are presented in Table 8. The highest iron values were obtained in steak, intermediate in shoulder and lower in ham. Results showed a significant (P<0.05) increase of iron content in C800 compared to the control S200 in all examined parts. Especially, in the ham the S800 group had significantly (P<0.05) higher iron content compared to the control S200 group.
Moreover, in the shoulder the C800 group had significantly (P< 0.05) higher iron levels compared to the S200 group. Regarding the other trace elements, Mo levels in ham tended (P< 0.10) to be higher in the chelated groups C200 and C800 compared to the both iron sulfate groups S200 and S800, but did not differ in the steak and shoulder parts. Zn, Se, Mn and B trace elements did not differ (P>0.05) between the groups in any examined tissues. Ba values could not be detected in either meat parts of the different groups. As and Cd values, although detected, their values were below quantification limit for any part of any different experimental group.
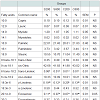
Meat fatty acid profile: Fatty acids content of pork meat of the steak, shoulder and ham parts are presented in Tables 10-12, respectively. The major fatty acids in pork meat of all groups were the oleic (C18:1) and thepalmitic (C16:0). Other abundant fatty acids were stearic (C18:0) and linoleic (C18:2). These fatty acids accounted for more than 80% of the total fatty acids in pork meat of the three analysed parts. In the steak, the C800 group had significantly (P< 0.05) lower capric (C10:0), lauric (C12:0), myristic (C14:0), myristoleic (C14:1), gamma-linolenic (C18:3n-6), arachidonic (C20:4n-6), andnervonic (C24:1n-9) levels compared to the control S200 group, although total saturated; monounsaturated and polyunsaturated fatty acids did not differ among the experimental groups. In the shoulder, it was noted that myristoleic (C14:1) fatty acid was significantly (P< 0.05) higher in the S200, compared to the C200 group. In the ham, trans-linoleic (6 trans-C18:2) was significantly (P< 0.001) lower in S800, C200 and C800 compared to the control S200 group, gammalinolenic was significantly (P< 0.05) lower in S800 group compared to the C200 and eicosenoic (C20:1n-9) was higher in the S200 group compared to the S800 and C200 groups.
Effect of Dietary Iron Sulfate and Iron Chelate on Growth Performance, Hematological Traits, Intestinal Microbial Flora of Fattening Pigs and Quality Parameters of Porkmeat
Ilias Giannenas1, Ioannis Skoufos2, Eleftherios Bonos1, Ioannis Sarakatsianos3, Athina Tzora2, Stylianos Skoufos2, Efterpi Christaki1e1 and Panagiota Florou-Paneri1*
- 1Laboratory of Nutrition, School of Veterinary Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece.
- 2Department of Animal Production, Technological Institute of Epirus, 47132, Arta, Greece.
- 3Third Military Veterinary Hospital, 57 001 Thermi, Thessaloniki, Greece.
*Address for Correspondence: Panagiota Florou-Paneri, Laboratory of Nutrition, School of Veterinary Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece, Tel: (+30) 2310999968; Fax: (+30) 2310999984; E-mail: ppaneri@vet.auth.gr
Citation: Giannenas I, Skoufos I, Bonos E, Sarakatsianos I, Tzora A, et al. Effect of Dietary Iron Sulfate and Iron Chelate on Growth Performance,Hematological Traits, Intestinal Microbial Flora of Fattening Pigs and Quality Parameters of Porkmeat. J Veter Sci Med. 2015;3(2): 11.
Copyright © 2015 Giannenas I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 3, Issue: 2
Submission: 29 September, 2015 | Accepted: 09 November, 2015 | Published: 14 November, 2015
Abstract
In this trial, 64 pigs with an average body weight of 54.0 kg and average age of 112 days were randomly allocated to 4 groups. The animals of the control group (S200) were fed with a standard commercial diet based on maize, barley and soybean meal in mash form which contained as source of iron 200 mg/kg iron sulfate. The diets of the other three groups, were identical with the only difference that they contained per kg either 800 mg iron sulfate (Group S800), or 200 mg iron chelate (Group C200), or 800 mg iron chelate (Group C800). All animals were reared in standard husbandry conditions in VIKI farm, Epirus, while feed and water were offered ad libitum. At the end of the trial (165th day of age), all animals were weighed, slaughtered and further processed. The results of the present study revealed that S800, C200 and C800 groups showed higher final body weight, compared to the control (S200) group. Hematocrit, hemoglobin, blood iron and ferritin content were also increased in the three experimental groups, compared to the controls. In the jejunum, total anaerobes and Clostridium perfringens counts were higher in groups S800 and C800, compared to the other two groups. The subcutaneous fat did not differ among the groups. C800 group had decreased total fat values in the steak, ham and shoulder meat parts, compared to the control. All meat parts of group C800 contained increased iron levels, compared to the control. Groups S800 and C800 had increased meat lipid oxidation values, compared to the other two groups after 1 day of refrigerated storage. Meat fatty acid profile did not significantly differ substantially among the groups. Supplementation with extra iron sulfate or iron chelate in swine nutrition improved slaughter weight and could be used as a dietary manipulation method to produce pork meat with improved chemical composition and desirable meat quality characteristics.Keywords
Iron chelate; Intestinal bacteria; Lipid oxidation; Fatty acid profile; ICP-MS trace element content; Blood traits.Introduction
Pork meat is a significant part of human diet in several parts of the world. Meat quality is especially important as the pork industry attempts to increase its presence in the global market and as it faces increased competition with red meat species or chicken meat. Supplementation of the swine diet with certain trace constituents such as trace elements, especially iron from inorganic or chelates sources during the growing and finishing periods may improve pork quality, as similar findings have been described for broilers [1,2]. In addition, meat is a well-known enhancer of non-heme iron bioavailability from foods, a fact commonly known as the ‘meat factor’ [3,4]. Thus, meat acts as both a source of heme iron and an enhancer of non-heme iron absorption in human diet.Meat consumption may be beneficial for people with iron deficiency, and could also serve as an excellent source of other essential trace elements such as Zn and Se [5]. Similarly, meat either from monogastric animals like pork or from ruminants like beef and lamb is a rich source of valuable and essential nutrients, such as high quality proteins, niacin, vitamins B1, B2, B3, B12 and certain trace elements [4]. Menstruating women in particular constitute a group at risk from iron deficiency. Surveys carried out in France and North America reported iron deficiency in nearly 20% of these women. This high prevalence was explained by inadequate dietary iron intake to compensate for iron losses in the menses [6]. Similarly, in Denmark, low iron status is common in partly breast-fed infants, and low iron stores have been documented in approximately 30% of women of fertile ages [7]. This inadequate dietary iron intake might be prevented by consumption of the readily available heme iron present in meat [8-10].
Accordingly, meat fortified with iron can be a novel, high value food especially for iron deficient consumers. A possible way to increase meat iron content is through extra dietary supplementation during the growing-fattening period of the animals. In addition, in recent years there is increased research interest in using chelated sources of iron, substituting in part or in total traditional sulfate iron sources.
It is possible that chelated or proteinated sources of iron have higher availability, compared with inorganic sources [2,11]. Iron fortification in pig diets, may also have positive effects on their health status and growth performances. Some previous studies showed that dietary supplementation with chelated iron improved growth performance, hematological and immunological characteristics, iron tissue storage, and antioxidant enzyme activity in weanling pigs [11,12]. In contrast, it has also been noted that dietary chelated iron did not affect feed intake, feed/gain ratio, blood immunoglobulins concentration, and B lymphocyte proliferation in weanling piglets [12]. Nevertheless, in fattening pigs, there is a scarce data, regarding the effect of chelated iron sources that could potentially improve both growth performance and iron storage in the meat, although it has been reported that replacing inorganic minerals with organic sources had no effect on several growth performance parameters of poultry [13].
Free iron in meat or increased dietary iron may accelerate the auto-oxidation of oxymyoglobin [14-16], the photo-oxidation of oxymyoglobin [17], the oxidation of reducing agents; e.g. cysteine, glutathione, ascorbate, tocopherols reducing the anti-oxidative capacity of the muscle [18] and the propagation phase in lipid peroxidation [15,16,19]. Ahn and Kim showed that in oil emulsions and cooked-meat homogenates, iron had a strong pro-oxidant effect and was the most important pro-oxidant among all iron sources measured [20]. Consequently, free iron either directly or indirectly may stimulate quality deterioration of meat through acceleration of the oxidation of oxymyoglobin to metmyoglobin or lipid peroxidation.
The objective of this experiment was to evaluate the effects of two iron sources (iron sulfate or iron chelate) of increasing levels, i.e. normal recommendation and high supplementation (fourfold) on the growth performance, hematological status and several carcass characteristics such as lipid oxidation, fatty acid profile, and trace element content of steak, ham and shoulder meat, as well as on intestinal microbiotain the jejunum, caecum and mid colon of fattening pigs.
The hypothesis was that dietary iron fortification would improve the growth performance of pigs, increase meat iron content, favor specific bacteria species in the intestine and improve fatty acid profile without negative effects on the antioxidant capacity of their tissues.
Materials and Methods
In this trial, 64 pigs ([Large White ×Landrace]×Duroc) of mixed sex with an average body weight of 54.0 kg and average age of 112 days were randomly allocated to 4 groups (2 males and 2 females per pen; 4 pens per treatment). All animals were reared in standard husbandry conditions (slatted plastic floors, density, humidity, temperature, ventilation), while feed and water were offered ad libitum.
The animals of the control group (Group S200) were fed with a standard commercial diet based on maize, barley and soybean meal in mash form, formulated to meet National Research Council recommendations [21]. This diet contained 200 mg /kg iron sulfate as source of iron. Table 1 presents the ingredients and the composition of the control diet. Proximate analysis of the diet was performed according to AOAC [22] for dry matter (Method 930.15), crude protein (Method 976.05), ether extract (Method 920.39), crude fiber (Method 978.10) and ash (Method 942.05). Major and trace element of the diet were determined using inductively coupled plasma mass spectrometry (ICP-MS) according to Nisianakis et al. [23]. Proximate analysis of three batches from this diet showed no major deviation from calculated values.
The trial protocol was approved by the Institutional Committee for Animal Use and Ethics of the Technological Institute of Epirus, Department of Agriculture Technology, Division of Animal Production. Throughout the trial, the pigs were handled in compliance with EU and National laws and regulations and in accordance to the principles and guidelines for the care of animals in experimentation [24].
Determination of blood constituents
At the end of the trial, blood samples were collected and hematocrit (Hct), hemoglobin (Hb), and percentage of lymphocytes, mononuclear and poly-morphonuclear cells were determined by a Hematology Analyzer MS4 Melet Schcoesin FG®, France. Iron was determined by the guanidine/FerroZine method by absorption at 552 nm, while ferritin was determined by ELISA and transferrin was determined in blood plasma of different groups according to Rincker et al. [25].
Enumeration of bacteria populations in jejunum, caecum and mid-colon
To determine bacteria populations, fresh weighed digesta samples from jejunum, mid colon and caecum were collected during slaughter and mixed homogeneously at a ratio of 1 g sample with 9 ml of peptone water (0.1% v/v) in the universal container for the enumeration of bacteria such as total aerobes, total anaerobes, total Coliforms, Clostridium perfringens, Enterococcus spp., Enterobacteriaceae, Lactobacillus spp. and Bifidobacterium spp. by conventional microbiological techniques using selective agar media [26]. Subsequently, serial decimal dilutions were made, avoiding aeration. Aerobes were enumerated using Plate Count Agar; the inoculated plates were incubated aerobically for up to 48 hours at 37 °C. Anaerobes were enumerated by using Plate Count Agar; the inoculated plates were incubated anaerobically (in jar) for up to 48 hours. For the determination of Lactobacillus spp., the samples plated onto de Man Rogosa Sharpe (MRS) agar and incubated under anaerobic conditions at 37 °C for 48 h. Bifidobacterium spp. were anaerobically assayed using Reinforced Clostridial Agar (RCA). Enterococci spp. were enumerated using Slanetz & Bartley Agar (aerobial incubation at 37 °C for 48 h). Clostridium perfringens enumeration was based on Tryptone Sulfite Cyclocerine Agar (TSC). For the detection and enumeration of Enterobacteriaceae the Vilet Red Bile Glucose (VRBG) agar was used. Samples incubated under aerobic conditions at 37 °C for 24 h on MacConkey agar for the determination of total coliform numbers. These processes were repeated twice and the results were expressed as colony forming units (CFU) per gram of sample (CFU/g).
Carcass quality characteristics
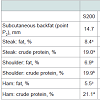
Subcutaneous fat: The subcutaneous fat (point P2) was measured by Minitube® Βack fat measuring device in all animals, one day before slaughter.
Meat chemical analysis: For each group parts of steak, ham and shoulder meat were taken from 16 animals (4 animals from each replication) and were analyzed for fat and protein content, by NIR spectroscopy using a Food ScanTM Lab, (FOSS, Denmark). For all samples, the visible extramuscular fat was removed with extensive trimming and then 200 g samples were minced (Cutter K35, Electrolux) and placed in the instrument tray for analysis. For the steak the eye part i.e. Longissimus dorsi muscle was used. For the leg parts, hams were cut, the Biceps femoris muscles were removed, and then all intermuscular fat and external connective tissue (perimysium) were trimmed. For the shoulder the Supraspinatus and the Infraspinatus muscles were used.
Meat major and trace element analysis: Certain trace or major elements were determined in feed and meat samples, using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7500s, Agilent Technologies, Waldbronn, Germany) according to Nisianakis et al. [23]. Samples were treated in triplicates and each sample was measured three times. The instrumental settings and operative conditions were: Frequency 27.12 MHz; Reflect power 1.55 kW; Reflect matching 1.62 V; Sampling depth 6.8 mm; Torch-H 0.1 mm; Torch-V 0.3 mm; Carrier gas 1.20 L/min; Nebuliser pump 0.10 rps; S/C temperature 2 ºC; Oxide ions 0.67% (156/140); Doubly charged 1.6 (70/140); Nebuliser type concentric. Recovery and detection limits of the analytical methodology used in the current study are presented in Table 3.
The major elements calcium (Ca) magnesium (Mg), potassium (K), sodium (Na) and trace elements iron (Fe), selenium (Se), zinc (Zn), manganese (Mn), copper (Cu), cobalt (Co), molybdenum (Mo), boron (B), barium (Ba), arsenic (As), lead (Pb) and cadmium (Cd) were determined in diets and trace elements iron (Fe), selenium (Se), zinc (Zn), manganese (Mn), molybdenum (Mo), borion (B), barium (Ba), arsenic (As) and cadmium (Cd) were determined in meat samples. Feed samples were collected prior to feeding and milled through a 1 mm sieve.
For digestion aliquots of 1 g homogenized samples were accurately weighed using a Teflon vessel. After the addition of 8 ml of concentrated HNO3 (65%) and 2 ml of H2O2 30% w/w, the digestion vessel was closed and heated in the microwave digestion system. The temperature was increased gradually up to 200 ºC in 10 min and remained constant for another 10 min. The obtained solutions were allowed to cool at room temperature, and were quantitatively transferred into a glass volumetric flask of 50 ml (class A) and completed to volume with with ultrapure deionised water. Analysis was performed by ICP-MS, following external calibration. Filtration was not necessary since the resultant digesta was clear enough.
Standard solutions were obtained from High Purity Standards (Merck, Germany), single element solutions (Ca, Mg, Fe, Se, Zn, Mn, Co, Cu, Mo, Cr, Ni, As and Cd) and used to get calibration curves. Several certified reference materials (Inorganic Ventures, Christiansburg USA) were used to validate the analytical procedure; these included the standard element solutions. All chemicals used were of analytical-grade. Nitric acid (Hiperpur) was purchased from Merck and internal standards (Sc, In, Ge, Bi) from Agilent.
Meat lipid oxidation: Moreover, lipid oxidation of raw meat during refrigerated storage, was determined as malondialdehyde (MDA) levels, using a modified version described by Florou-Paneri et al. [27]. The previously frozen samples were thawed overnight at 4 ºC placed in a non-illuminated refrigerated cabinet, minced using a commercial food processor, wrapped in oxygen-permeable film and stored at 4 ºC for a total of 9 days. On the 5th and the 9th refrigeration days, from each sample, subsamples were taken and processed. Absorbance was read at 532 nm against a blank sample using an UVVIS spectrophotometre (UV-1700 PharmaSpec, Shimadzu, Japan) 1,1,3,3 tetraethoxypropane was used as standard and results were expressed as ng of MDA per g of sample.
Meat fatty acid profile: The fatty acid composition of the steak, ham and shoulder portions was determined by gas chromatography. Fatty acids methyl esters were obtained from the frozen samples using the protocol described by O’Fallon et al. [28]. Then, the separation and quantification of the methyl esters was carried out with a gas chromatographic system (TraceGC model K07332, ThermoFinnigan, ThermoQuest, Milan, Italy) equipped with a flame ionization detector, a model CSW 1.7 chromatography station (CSW, DataApex Ltd, Prague, Czech Republic) and a fused silica capillary column, 30 m x 0.25 mm i.d., coated with cyanopropylpolysiloxane (phase type SP- 2380) with a film thickness of 0.20 μm (Supelco, Bellefonte, PA, USA). The chromatographic conditions were: Carrier: N2 , Flow: 1 ml/min; Oven: Temperature 70 ºC for 0.5 min, increase 30 ºC/min to 180 ºC for 10 min, increase 5 ºC/min to 225 ºC for 15 min; Inlet temperature: 250 ºC; Detector temperature: 250 ºC; Injection: 1 μl, with split 1/20. Fatty acid methylesters retention times and elusion order were identified using as reference standards the Supelco ‘F.A.M.E Mix C8-C24’ (C.N. 18918-1AMP), the Supelco ‘37 Component FAME Mix’ (47885-U), the Supelco ‘Linoleic acid methyl ester cis/trans isomers’ (4-7791) and the Sigma ‘Tridecanoic acid’ (T0502-5G), as well as accompanying Supelco reference material for the procedure. Fatty acids were quantified by peak area measurement and the results were expressed as percentage (%) of the total peak areas for all quantified acids.
Statistical analysis
For the statistical evaluation of the experimental study results, data were subjected to analysis of variance (ANOVA), using the statistical package of IBM SPSS Statistics v. 21.0 Statistical Package (SPSS Inc., Chigaco, IL, USA). Tukey’s multiple range test was used to distinguish the statistical difference among the mean value of each experimental group participated in the trial. The level of significance was set at 5% (α=0.05).
Results
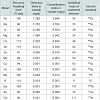
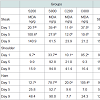
Animal performanceAt the end of the fattening period, high iron sulfate and both iron chelate supplemented groups had significantly (P< 0.001) higher body weight values compared to the control group supplemented with low level of iron sulfate (Table 4). Feed intake and feed conversion ratio (FCR) was not affected (P>0.05) by iron inclusion during the entire experimental period (Table 4). There was no mortality throughout the experiment.
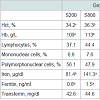
In the present study, Hct, Hb, blood iron and ferritin content were significantly (P< 0.05) increased in groups fed either the high iron sulfate level or the iron chelate diets (Table 5), however, transferrin values, lymphocytes, mononuclear and polymorphonuclear cells percentages were not affected by dietary iron fortification.
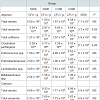
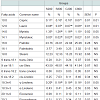
The composition of the intestinal microbiota of fattening pigs at slaughter is shown in Table 6. In the jejunum, the total anaerobes and Clostridium perfringens counts were higher (P< 0.05) in the pigs supplemented with high levels of dietary iron (S800 and C800) compared to pigs fed either the low sulfate S200 or the low chelate iron C200. In caecum or mid-colon no significant (P>0.05) differences were noted for the total anaerobes, total aerobes, total coliforms, enteroccococus, enterobacteriaceae, lactobacilli, bifidobacteria and Clostridium perfringens counts.
Subcutaneous fattened meat chemical analysis: The subcutaneous fat (point P2) did not differ (P>0.05) among the experimental groups (Table 7). The meat composition analysis showed that iron chelate dietary supplementation significantly decreased (P< 0.001) total fat in the steak, shoulder and ham parts, and increased protein content in the steak and the ham parts compared to the control group S200.
Meat lipid oxidation: Table 9 presents the effects of both sources and levels of iron supplementation and duration of refrigerated storage on tissue MDA levels. On day 1 of refrigerated storage, on the steak, shoulder and ham parts MDA levels were significantly (P< 0.05) higher for the iron chelate group C800, compared to the controls S200. On day 5 the S200 group had significantly (P< 0.05) higher MDA levels compared to the other groups in the steak, and in the ham group S800 tended (P< 0.10) to have higher MDA level compared to groups C200 and C800, but this effect was not found in the shoulder parts. On day 9, on the steak part group S200 tended (P< 0.10) to have higher MDA levels compared to groups C200 and C800. It should be noted that a wide variability of measurements was noted especially in the S800 and C800 groups.
Discussion
The present study was designed to evaluate the effects of two dietary iron sources (iron sulfate or iron chelate) supplemented at two levels on performance parameters and meat quality characteristics of fattening pigs.There is scarce information about the effects of dietary iron fortification in fattening pigs, although several investigations have been conducted either for nursery or weaned piglets or sows. Iron is recognized as one of the most important trace elements for animal growth [29], especially in swine. Iron is the first deficient trace element in piglets, especially during the first few weeks of life. Post weaning iron provided by common feed ingredients may meet most of the dietary requirement [25]. However, the bioavailability of iron from different sources varies greatly [30,31] and may be influenced by factors such as blood iron status of the animal, dietary iron concentration and other nutritional or non-nutritional elements within the diet [25].
Evidence exists that iron can exert growth promoting activities, as seen in past studies mainly focused on piglets although this effect is not consistent [11,12]. In our study, moderate iron fortification resulted in a considerable body weight increase of the pigs at slaughter. Similarly, Miller et al. found that daily gain of the slaughter pigs enhanced with increasing dietary iron intake [32].
Moreover, the animals that consumed the high-level iron sulfate or iron chelate diet showed increased blood Hct, Hb, iron and ferritin. This is in accordance with the results of others studies on piglets which examined diets with either higher iron sulfate levels or iron chelate forms [33,34]. Rincker et al. also showed a linear increase in Hb, Hct, blood iron and plasma transferrin content after dietary iron fortification of nursery pigs for 35 days [25]. Ma et al. also reported that blood Hb concentration and total body Hb iron were sensitive indices in reflecting differences in bioavailability among different iron sources, and iron proteinate was significantly more available to animal than inorganic iron sulfate in enhancing Hb concentration and total body Hb iron [1]. Similarly, supplementation of swine diets with iron altered muscle total, heme, and non-heme iron concentrations [9,34,35]. However, Apple et al. did not find an increase in Hbiron values after iron supplementation [36]. Miller et al. observed that non-heme iron concentrations of fresh M. longissimus and Rectus femoris were similar among pigs fed diets containing 62, 131, or 209 ppm Fe [37]. It should be also noted that elevated total M. longissimus iron concentrations were observed after supplementation of swine finishing diets with 3,000 ppm iron from iron sulfate [9,35].
Plasma ferritin is a protein that stores iron and releases it in a controlled way. Plasma ferritin is circulating several times daily, so an iron atom typically remains no longer than 2 h in plasma; in addition, it exhibits a diurnal variation, with a decrease in concentration in the evening [25]. The observed ferritin increase in the dietary iron fortified groups was in agreement with previously reported results [25,34].
Another key facilitator in the maintenance of iron homeostasis is the plasma glycoprotein, transferrin, which is the primary form of inter organ transport of non-hemeiron [25]. Elevated transferrin concentration is associated with an increase in iron absorption from the gut or mobilization of iron from tissues stores. The abundance of plasma transferrin is inversely related to iron status because it is generally used to transport non-hemeiron due to demand by irondependent tissue in pigs [25]. In the present study, transferrin levels in the blood were not affected by extra iron fortification or by the different iron sources.
In the present study, although iron fortification was coupled with significant performance improvement, no major changes were noted for intestinal microbiota. Dietary iron fortification increased total anaerobes and Clostridium perfringens populations in the jejunum, but lactobacilli or bifidobacteria loads were not affected. Iron may be a significant factor for bacterial growth, but other factors such as feed energy and ingredients, and the age of the animals may also affect intestinal microbiota [38,39]. Establishment of beneficial microflora such as increased counts of lactobacilli and bifidobacteria spp. may improve gastrointestinal function, feed digestibility, animal performance and health [40]. In the present trial, although dietary iron supplementation improved growth performance, this finding cannot be attributed to an improved intestinal microflora composition, i.e. increased lactobacilli or bifidobacteria counts.
The second objective of this study was to investigate whether the iron fortification would benefit the meat quality characteristics. Subcutaneous backfat was not influenced by dietary treatment, however total fat in steak and shoulder was reduced in the groups supplemented with iron chelate. Scarce information is available concerning the effects of dietary iron on pork carcass composition or pork meat quality. Saddoris et al. observed that supplementing swine diets with 90 ppm iron from either chelate or sulfate sources did not affect average backfat depth or M. longissimus area [41]. Apple et al. supplemented swine diets with iron and noticed no effect on M. longissimus moisture content or drip loss percentage, slaughter and hot carcass weight [36]. Moreover, dressing percentage, fat depth, M. longissimus depth and area, and fat free lean yield, were similar between carcasses of pigs fed either low or high iron supplemented diets [36]. In this study, only a tendency for 10th-rib fat depth to increase and fat-free lean yield to decrease linearly as dietary iron increased from 50 to 150 ppm was found [36].
An important hypothesis in this trial was to examine if increased dietary iron levels can elevate meat iron concentration, as well as to examine the effect on the content of other meat trace minerals. Until recently, there have been some difficulties with the estimation of the micro mineral composition in meat and other animal food products; the used methodologies i.e. atomic absorption spectroscopy or photometric methods, had been either time consuming and costly, or more importantly did not allow simultaneous estimation of the micro minerals concerned [23]. The inductively coupled plasma mass spectrometry (ICP-MS) is well established as a method for multi elemental analysis and the determination of isotope ratios [23,42,43], and overcomes many of these problems. This methodology allows simultaneous analysis of a wide range of trace elements in the same sample and has been used in this study.
Increased iron content in the feed diet resulted in increased meat iron in all examined tissues (steak, ham, shoulder) especially for the iron chelate form. Fourfold increase in dietary iron was correlated with about double iron meat content in ham. These findings are in agreement with Miller et al. who studied pigs fed either, 62, 131 or 209 ppm iron, and found that non-heme iron concentrations in M. longissimus dorsi and M. rectus femoris increased with dietary iron level [37]. Similar to our results, several workers found that iron concentration varied between different cuts from the same species [44-46]. The iron values, can vary significantly among different parts of pork meat from different groups and this can be further affected by breed or feeding system [46].
In this study, the other examined trace minerals Mo, Zn, Se, Mn and B did not differ in any of the examined tissues, among the experimental groups. Similarly, Rincker et al. also showed that the total body iron content linearly increased after dietary iron fortification; however no differences were observed in total body Cu, Zn, Mg, Mn, Ca and P levels after 35 days of higher iron supplementation [25]. In our study, Zn values did not differ greatly between the different meat parts. In contrast, other studies showed that Zn can differ substantially between different meat parts [44,47]. Cassens et al. reported that the Zn content in various porcine parts varied with color and myoglobin concentrations and that dark muscles had greater Zn concentrations than light ones; they also found increased Zn content in more active muscles [48].
Although, meat is known to be a source of essential trace elements, it can also accumulate toxic heavy metals such as Cd, As or Pb. In the present study, content of toxic trace elements were not detected. In general, the content of toxic elements in meat is rare, whereas offal such as liver, kidneys and intestines often accumulate higher concentrations [46,49].
In this study, it was noted that the groups that were fed elevated levels of either iron sulfate or chelate, had increased lipid oxidation values in the steak, ham and shoulder meat on the first day of refrigerated storage. The measured MDA values were lower in the 5 and 9 day of storage and did not differ between the groups. This declining trend in oxidation products as dark storage progresses is in agreement with previous published data in ham [50,51] and can be attributed to the instability or transitory nature of MDA. It can be hypothesized that higher MDA levels after slaughter and meat processing was attributed to the increased iron content in all meat parts. The literature is abundant with evidence of in vitro and in vivo oxidative activity of iron [15,16,52]. Increased tissue iron content, is accompanied by enhanced ability of free radicals to promote MDA formation [53]. The increased oxidation values in iron fortified groups, can possibly be correlated with the high heme iron and myoglobin contents of these muscles [54,55] suggesting that this meat may be more prone to lipid oxidation [16,57,58].
Previous studies have demonstrated the advantages of dietary vitamin such as vitamin E or C, supplementation relative to pig growth traits, color and flavor characteristics of fresh pork, and drip loss of pork [9,58]. It could be speculated that extra dietary ascorbic acid or a-tocopherol supplementation is needed to improve oxidativestability of pork meat with higher iron content.
Fatty acid profile for total saturated, monounsaturated and polyunsaturated fatty acids in the examined meat parts of the experimental groups was not affected by the different sources of iron or the levels of supplementation. Fatty acid contents in the examined pork meat were comparable to previously published values [16], however data to compare fatty acid profile in high iron supplemented pigs are scarce in the literature. It has been suggested that the meat fatty acid composition could be a predictor for the oxidation stability of the product [59,60]. In the present study, the observed increase in meat iron content was not accompanied by a modification of meat unsaturated fatty acid profile. Previous literature data about the effect of dietary iron fortification on meat fatty acid content were not available for further comparisons.
Conclusion
The swine industry is continually looking for methods to improve the quality and consumer acceptability of pork meat. The results presented in this paper suggest that iron fortification may be able to improve both growth performance and certain blood trait characteristics, while major effects were not noted in intestinal microbiota. Iron meat content was also positively increased; however oxidative stability of the produced pork meat was reduced in refrigerated storage. Further research is required to stabilize the possible effects of dietary iron fortification and to minimize the detrimental effects, especially on lipid oxidation.Acknowledgements
This research was funded from EEC and National Funds, under the research program Bilateral R&T Cooperation Greece-China, 2012–2014 (EPAN II-EII--12CHN91-GreenPork).References
- Ma XY, Liu SB, Lu L, Li SF, Xie JJ, et al. (2014) Relative bioavailability of iron proteinate for broilers fed a casein-dextrose diet. Poult Sci 93: 556-563.
- Shi R, Liu D, Sun J, Yia Y, Zhang P (2015) Effect of replacing dietary FeSO4 with equal Fe-levelled iron glycine chelate on broiler chickens. Czech J Anim Sci 60: 233-239.
- Mulvihill B, Morrissey PA (1998) Influence of the sulphydryl content of animal proteins on in vitro bioavailability of non-haem iron. Food Chem 61: 1-7.
- Reig M, Aristoy MC, Toldra F (2013) Variability in the contents of pork meat nutrients and how it may affect food composition databases. Food Chem 140: 478-482.
- Briggs GM, Schweigert BS (1990) An overview of meat in the diet. In: Pearson AM, Dutson TR, (eds). Meat and health: Advances in meat research, London: Elsevier Applied Science 6: 1-18.
- Fricker J, LeMoel G, Apfelbaum M (1990) Obesity and iron status in menstruating women. Am J Clin Nutr 52: 863-866.
- Milman N, Kirchoff M (1992) Iron stores in 1396, 30- to 60- year-old Danish women: evaluation by serum ferritin and haemoglobin. Ann Hematol 64: 22-27.
- Leonhardt M, Kreuzer M, Wenk C (1997) Available iron and zinc in major lean meat cuts and their contribution to the recommended trace element supply in Switzerland. Nahrung 41: 289-292.
- O' Sullivan MG, Byrne DV, Nielsen JH, Andersen HJ, Martens M (2003) Sensory and chemical assessment of pork supplemented with iron and vitamin E. Meat Sci 64: 175-189.
- McArthur JO, Gough NM, Petocz P, Samman S (2014) Inclusion of pork meat in the diets of young women reduces their intakes of energy-dense, nutrient-poor foods: results from a randomized controlled trial. Nutrients 6: 2320-2332.
- Feng J, Ma WQ, Xu ZR, He JX, Wang YZ, et al. (2009) The effect of iron glycine chelate on tissue mineral levels, fecal mineral concentration, and liver antioxidant enzyme activity in weanling pigs. Anim Feed Sci Technol 150: 106-113.
- Feng J, Ma WQ, Xu ZR, Wang YZ, Liu JX (2007) Effects of iron glycine chelate on growth, haematological and immunological characteristics in weanling pigs. Anim Feed Sci Technol 134: 261-272.
- Nollet L, van der Klis JD, Lensing M, Spring P (2007) The effect of replacing inorganic with organic trace minerals in broiler diets on productive performance and mineral excretion. J Appl Poult Res 16: 592-597.
- Snyder HE, Skrdlant HB (1966) The influence of metallic ions on the autoxidation of oxymyoglobin. J Food Sci 31: 468-473.
- Barriuso B, Astiasaran I, Ansorena D (2012) A review of analytical methods measuring lipid oxidation status in foods: a challenging task. Eur Food Res Technol 236: 1-15.
- Karwowska M, Dolatowski ZJ (2013) Comparison of lipid and protein oxidation, total iron content and fatty acid profile of conventional and organic pork. Int J Food Sci Technol 48: 2200-2206.
- Assaf SA, Bratzler LJ, Cameron BF, Yunis AA (1971) Photo-oxidation of bovine oxymyoglobin in frozen solutions. The effect of redox active inorganic elements in muscle extracts. Comp Biochem Physiol B 39: 395-407.
- Kanner J, Hazan B, Doll L (1988) Catalytic “free” iron in muscle foods. J Agric Food Chem 36: 412-415.
- Harel S, Kanner J (1985) Muscle membranal lipid peroxidation initiated by H2O2-activated metmyoglobin. J Agric Food Chem 33: 1188-1192.
- Ahn DU, Kim SM (1998) Prooxidant effects of ferrous iron, haemoglobin, and ferritin in oil emulsion and cooked meat homogenates are different from those in raw-meat homogenates. Poult Sci 77: 348-355.
- NRC (2012) Nutrient Requirements of Swine: Eleventh Revised Edition. Washington: D.C, USA: National Academy Press.
- AOAC (2005) Official Methods of Analysis. Arlington, Virginia, USA: Association of Analytical Chemists, AOAC International.
- Nisianakis P, Giannenas I, Gavriil A, Kontopidis G, Kyriazakis I (2009) Variation in trace element contents among chicken, turkey, duck, goose, and pigeon eggs analyzed by inductively coupled plasma mass spectrometry (ICP-MS). Biol Trace Elem Res 128: 62-71.
- NRC (1996) Guide for the care and use of laboratory animals. Washington, DC: National Academy Press.
- Rincker MJ, Hill GM, Link JE, Rowntree JE (2004) Effects of dietary iron supplementation on growth performance, hematological status, and whole-body mineral concentrations of nursery pigs. J Anim Sci 82: 3189-3197.
- Barrow GI, Feltham RK (1993) Cowan and steel’s manual for the identification of medical bacteria. Cambridge University Press, Cambridge.
- Florou-Paneri P, Palatos G, Govaris A, Botsoglou D, Giannenas I, et al. (2005) Oregano herb versus oregano essential oil as feed supplements to increase the oxidative stability of turkey meat. Int J Poult Sci 4: 866-871.
- O'Fallon JV, Busboom JR, Nelson ML, Gaskins CT (2007) A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils and feedstuffs. J Anim Sci 85: 1511-1521.
- Brock JH (1994) Iron in infection, immunity, inflammation and neoplasia. In: Brock JH, Halliday JW, Pippard MJ, Powell LW, (eds). Iron metabolism in health and disease, London: Saunders. pp. 354-389.
- Deming JG, Czarnecki-Maulden GL (1989) Iron bioavailability in calcium and phosphorus sources. J Anim Sci 67: 253.
- Cao J, Luo XG, Henry PR, Ammerman CB, Littell RC, et al. (1996) Effect of dietary iron concentration, age, and length of iron feeding on feed intake and tissue iron concentration of broiler chicks for use as a bioassay of supplemental iron sources. Poult Sci 75: 495-504.
- Miller DK, Smith VL, Kanner J, Miller DD, Lawless HT (1994) Lipid oxidation and warmed-over aroma in cooked ground pork from swine fed increasing levels of iron. J Food Sci 59: 751-756.
- Dove CR, Haydon KD (1991) The effect of copper addition to diets with various iron levels on the performance and hematology of weanling swine. J Anim Sci 69: 2013-2019.
- Yu B, Huang WJ, Chiou PW (2000) Bioavailability of iron from amino acid complex in weaning pigs. Anim Feed Sci Technol 86: 39-52.
- O' Sullivan MG, Byrne DV, Jensen MT, Andersen HJ, Vestergaard J (2003) A comparison of warmed-over flavour in pork by sensory analysis, GC/MS and the electronic nose. Meat Sci 65: 1125-1138.
- Apple JK, Wallis-Phelps WA, Maxwell CV, Rakes LK, Sawyer JT, et al. (2007) Effect of supplemental iron on finishing swine performance, carcass characteristics, and pork quality during retail display. J Anim Sci 85: 737-745.
- Miller DK, Gomez-Basauri JV, Smith VL, Kanner J, Miller DD (1994) Dietary iron in swine rations affects nonheme iron and TBARS in pork skeletal muscles. J Food Sci 59: 747-750.
- Kortman GA, Raffatellu M, Swinkels DW, Tjalsma H (2014) Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev 38: 1202-1234.
- Kortman GA, Boleij A, Swinkels DW, Tialsma H (2012) Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS One 7: e29968.
- Rehman HU, Vahjen W, Awad WA, Zentek J (2007) Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch Anim Nutr 61: 319-335.
- Saddoris KL, Crenshaw TD, Claus JR, Fakler TM (2003) Growth performance, carcass characteristics, and pork color in finishing pigs fed two sources of supplemental iron. J Anim Sci 81 Suppl 2: 69.
- Giannenas I, Nisianakis P, Gavriil A, Kontopidis G, Kyriazakis I (2009) Trace mineral content of conventional, organic and courtyard eggs analysed by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem 114: 706-711.
- Date AR, Gray AL (1983) Development progress in plasma source mass spectrometry. Analyst 108: 159-165.
- Schricker BR, Miller DD, Stouffer JR (1982) Measurement and content of nonheme and total iron in muscle. J Food Sci 47: 740-743.
- Carpenter CE, Clark E (1995) Evaluation of methods used in meat iron analysis and iron content of raw and cooked meats. J Agric Food Chem 43: 1824-1827.
- Gerber N, Brogioli R, Hattendorf B, Scheeder MR, Wenk C, et al. (2009) Variability of selected trace elements of different meat cuts determined by ICP-MS and DRC-ICPMS. Animal 3: 166-172.
- Marchello MJ, Slanger WD, Milne DB (1985) Macro and micro minerals from selected muscles of pork. J Food Sci 50: 1375-1378.
- Cassens RG, Briskey EJ, Hoekstra WG (1963) Variation in zinc content and other properties of various porcine muscles. J Sci Food Agric 14: 427-432.
- EFSA (2006) Tolerable upper intake levels for vitamins and minerals. Brussels: European Food Safety Authority.
- Gokalp HY, Ockerman HW, Plimpton RF, Harper WJ (1983) Fatty acids of neutral and phospholipids, rancidity scores and TBA values as influenced by packaging and storage. J Food Sci 48: 829-834.
- Haile DM, De Smet S, Claeys E, Vossen E (2013) Effect of light, packaging condition and dark storage durations on colour and lipid oxidative stability of cooked ham. J Food Sci Technol 50: 239-247.
- Min B, Ahn DU (2005) Mechanism of lipid peroxidation in meat and meat products - A review. Food Sci Biotechnol 14: 152-163.
- Hirano R, Sasamoto W, Matsumoto A, Itakura H, Igarashi O, et al. (2001) Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J Nutr Sci Vitaminol (Tokyo) 47: 357-362.
- Alasnier C, Meynier A, Viau M, Gandemer G (2000) Hydrolytic and oxidative changes in the lipids of chicken breast and thigh muscles during refrigerated storage. J Food Sci 65: 9-14.
- Min B, Nam KC, Cordray J, Ahn DU (2008) Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J Food Sci 73: C439-C446.
- Hansen LL, Claudi-Magnussen C, Jensen SK, Andersen HJ (2006) Effect of organic pig production systems on performance and meat quality. Meat Sci 74: 605-615.
- Lairon D (2010) Nutritional quality and safety of organic food. A review. Agron Sustain Dev 30: 33-41.
- Cannon JE, Morgan JB, Schmidt GR, Tatum JD, Sofos JN, et al. (1996) Growth and fresh meat quality characteristics of pigs supplemented with vitamin E. J Anim Sci 74: 98-105.
- Yun JM, Surh J (2012) Fatty acid composition as a predictor for the oxidation stability of Korean vegetable oils with or without induced oxidative stress. Prev Nutr Food Sci 17: 158-165.
- Narciso-Gaytan C, Shin D, Sams AR, Keeton JT, Miller RK, et al. (2011) Lipid oxidation stability of omega-3- and conjugated linoleic acid-enriched sous vide chicken meat. Poult Sci 90: 473-480.